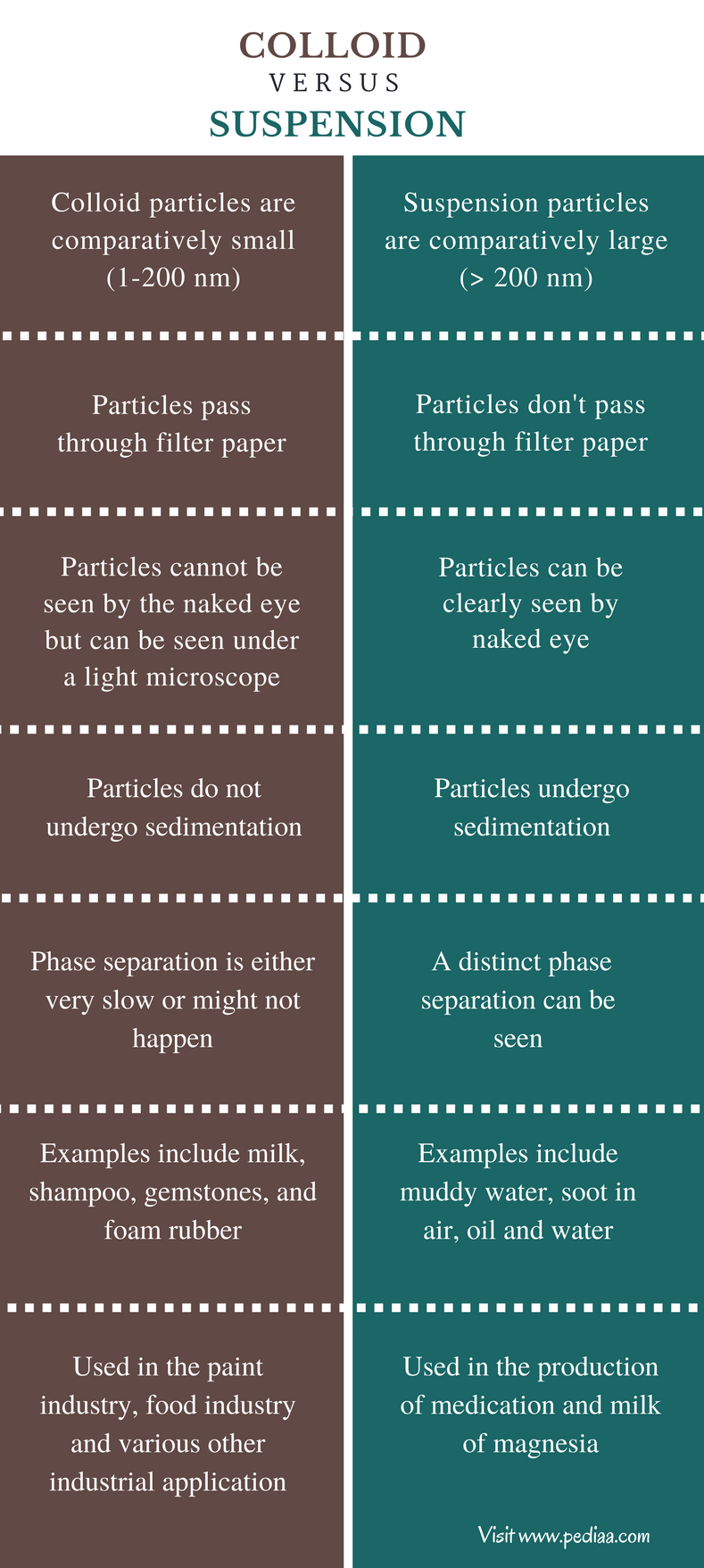
Difference Between Suspension And Colloid Solution . The particles in a solution are much. Colloid particles are much smaller than suspension particles. The very first difference between the two. Let’s explore some of the primary differences between colloids and suspensions. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. The true solution is the homogenous mixture, while colloidal solution and suspension are the heterogeneous mixtures of two or more substances. The main difference between colloid and suspension lies in the size of particles. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other substance. Another difference between these three. Due to this size difference, colloid particles. Difference between suspension and colloid. The solute particles in the true solution do not settle.the solute particles in the colloidal solution can be made to settle by centrifugation.the solute.
from pediaa.com
Difference between suspension and colloid. Let’s explore some of the primary differences between colloids and suspensions. The true solution is the homogenous mixture, while colloidal solution and suspension are the heterogeneous mixtures of two or more substances. The particles in a solution are much. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. The very first difference between the two. Due to this size difference, colloid particles. The solute particles in the true solution do not settle.the solute particles in the colloidal solution can be made to settle by centrifugation.the solute. Another difference between these three. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other substance.
Difference Between Colloid and Suspension Definition, Properties
Difference Between Suspension And Colloid Solution Another difference between these three. Let’s explore some of the primary differences between colloids and suspensions. Another difference between these three. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other substance. Due to this size difference, colloid particles. Difference between suspension and colloid. The solute particles in the true solution do not settle.the solute particles in the colloidal solution can be made to settle by centrifugation.the solute. The main difference between colloid and suspension lies in the size of particles. The particles in a solution are much. Colloid particles are much smaller than suspension particles. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. The very first difference between the two. The true solution is the homogenous mixture, while colloidal solution and suspension are the heterogeneous mixtures of two or more substances.
From pediaa.com
Difference Between Colloid and Solution Definition, Properties Difference Between Suspension And Colloid Solution Colloid particles are much smaller than suspension particles. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. The main difference between colloid and suspension lies in the size of particles. The true solution is the homogenous mixture, while colloidal solution and suspension are the heterogeneous mixtures. Difference Between Suspension And Colloid Solution.
From www.studypage.in
True Solution, Colloidal Solution and Suspension Difference Between Suspension And Colloid Solution A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other substance. The true solution is the homogenous mixture, while colloidal solution and suspension are the heterogeneous mixtures of two or more substances. The very first difference between the two. Let’s explore some of the primary differences between colloids and suspensions.. Difference Between Suspension And Colloid Solution.
From www.slideserve.com
PPT Chapter 12 Solutions PowerPoint Presentation, free download ID Difference Between Suspension And Colloid Solution A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other substance. Let’s explore some of the primary differences between colloids and suspensions. Due to this size difference, colloid particles. The particles in a solution are much. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in. Difference Between Suspension And Colloid Solution.
From ar.inspiredpencil.com
Colloid Suspension Solution Difference Between Suspension And Colloid Solution Another difference between these three. The solute particles in the true solution do not settle.the solute particles in the colloidal solution can be made to settle by centrifugation.the solute. Due to this size difference, colloid particles. Colloid particles are much smaller than suspension particles. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically. Difference Between Suspension And Colloid Solution.
From www.slideserve.com
PPT Solutions PowerPoint Presentation, free download ID1980281 Difference Between Suspension And Colloid Solution A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other substance. The particles in a solution are much. The true solution is the homogenous mixture, while colloidal solution and suspension are the heterogeneous mixtures of two or more substances. Let’s explore some of the primary differences between colloids and suspensions.. Difference Between Suspension And Colloid Solution.
From www.facebook.com
ChemEducation Difference between solution, suspension... Difference Between Suspension And Colloid Solution The main difference between colloid and suspension lies in the size of particles. The particles in a solution are much. Difference between suspension and colloid. The very first difference between the two. Let’s explore some of the primary differences between colloids and suspensions. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those. Difference Between Suspension And Colloid Solution.
From captionsfeaturenl.blogspot.com
what is the difference between a solution and a suspension Captions Difference Between Suspension And Colloid Solution Another difference between these three. Let’s explore some of the primary differences between colloids and suspensions. The solute particles in the true solution do not settle.the solute particles in the colloidal solution can be made to settle by centrifugation.the solute. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution. Difference Between Suspension And Colloid Solution.
From www.differencebetween.com
Difference Between Suspension and Colloid Compare the Difference Difference Between Suspension And Colloid Solution The true solution is the homogenous mixture, while colloidal solution and suspension are the heterogeneous mixtures of two or more substances. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other substance. The particles in a solution are much. The solute particles in the true solution do not settle.the solute. Difference Between Suspension And Colloid Solution.
From exoegsxur.blob.core.windows.net
Solution Suspension Colloid Quizlet at Mable Finger blog Difference Between Suspension And Colloid Solution Another difference between these three. Due to this size difference, colloid particles. The very first difference between the two. Let’s explore some of the primary differences between colloids and suspensions. The true solution is the homogenous mixture, while colloidal solution and suspension are the heterogeneous mixtures of two or more substances. Colloid particles are much smaller than suspension particles. The. Difference Between Suspension And Colloid Solution.
From www.slideshare.net
Unit 2, Lesson 2.5 Suspensions and Colloids Difference Between Suspension And Colloid Solution Difference between suspension and colloid. The very first difference between the two. The solute particles in the true solution do not settle.the solute particles in the colloidal solution can be made to settle by centrifugation.the solute. The main difference between colloid and suspension lies in the size of particles. A colloid is a mixture in which one of the soluble. Difference Between Suspension And Colloid Solution.
From www.differencebetween.com
Difference Between Colloid and Emulsion Compare the Difference Difference Between Suspension And Colloid Solution The particles in a solution are much. The very first difference between the two. The main difference between colloid and suspension lies in the size of particles. Difference between suspension and colloid. Colloid particles are much smaller than suspension particles. The true solution is the homogenous mixture, while colloidal solution and suspension are the heterogeneous mixtures of two or more. Difference Between Suspension And Colloid Solution.
From loezijvab.blob.core.windows.net
Colloid Solution Definition at Gary Reese blog Difference Between Suspension And Colloid Solution The true solution is the homogenous mixture, while colloidal solution and suspension are the heterogeneous mixtures of two or more substances. The solute particles in the true solution do not settle.the solute particles in the colloidal solution can be made to settle by centrifugation.the solute. Let’s explore some of the primary differences between colloids and suspensions. The main difference between. Difference Between Suspension And Colloid Solution.
From byjus.com
What is difference between true solution and suspension and colloid? Difference Between Suspension And Colloid Solution Difference between suspension and colloid. The particles in a solution are much. The very first difference between the two. The true solution is the homogenous mixture, while colloidal solution and suspension are the heterogeneous mixtures of two or more substances. Colloid particles are much smaller than suspension particles. A colloid is a heterogeneous mixture in which the dispersed particles are. Difference Between Suspension And Colloid Solution.
From www.geeksforgeeks.org
Suspension(Chemistry) Definition, Properties, Examples, and FAQs Difference Between Suspension And Colloid Solution The main difference between colloid and suspension lies in the size of particles. Difference between suspension and colloid. The solute particles in the true solution do not settle.the solute particles in the colloidal solution can be made to settle by centrifugation.the solute. Another difference between these three. The particles in a solution are much. The true solution is the homogenous. Difference Between Suspension And Colloid Solution.
From 88guru.com
True solution, Suspension, Colloidal Solutions 88Guru Difference Between Suspension And Colloid Solution The very first difference between the two. Due to this size difference, colloid particles. Colloid particles are much smaller than suspension particles. Let’s explore some of the primary differences between colloids and suspensions. Another difference between these three. The true solution is the homogenous mixture, while colloidal solution and suspension are the heterogeneous mixtures of two or more substances. Difference. Difference Between Suspension And Colloid Solution.
From mungfali.com
True Solution Colloid And Suspension Difference Between Suspension And Colloid Solution The very first difference between the two. The particles in a solution are much. The true solution is the homogenous mixture, while colloidal solution and suspension are the heterogeneous mixtures of two or more substances. Difference between suspension and colloid. The solute particles in the true solution do not settle.the solute particles in the colloidal solution can be made to. Difference Between Suspension And Colloid Solution.
From www.youtube.com
Solution, Suspension and Colloid aumsum kids science education Difference Between Suspension And Colloid Solution Another difference between these three. The solute particles in the true solution do not settle.the solute particles in the colloidal solution can be made to settle by centrifugation.the solute. The very first difference between the two. Let’s explore some of the primary differences between colloids and suspensions. Due to this size difference, colloid particles. Difference between suspension and colloid. The. Difference Between Suspension And Colloid Solution.
From edurev.in
Difference between true solution, suspension and colloidal sol Difference Between Suspension And Colloid Solution The true solution is the homogenous mixture, while colloidal solution and suspension are the heterogeneous mixtures of two or more substances. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically. Difference Between Suspension And Colloid Solution.
From pediaa.com
Difference Between Colloid and Suspension Definition, Properties Difference Between Suspension And Colloid Solution The main difference between colloid and suspension lies in the size of particles. Colloid particles are much smaller than suspension particles. Due to this size difference, colloid particles. The very first difference between the two. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other substance. A colloid is a. Difference Between Suspension And Colloid Solution.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension Difference Between Suspension And Colloid Solution Let’s explore some of the primary differences between colloids and suspensions. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other substance. The main difference between colloid and suspension lies in the size of particles. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size. Difference Between Suspension And Colloid Solution.
From studylib.net
Solutions, Colloids, Suspension Difference Between Suspension And Colloid Solution Let’s explore some of the primary differences between colloids and suspensions. The main difference between colloid and suspension lies in the size of particles. The very first difference between the two. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Due to this size difference, colloid. Difference Between Suspension And Colloid Solution.
From www.pinterest.com
mixture suspension colloid Google Search STEM Physical Science Difference Between Suspension And Colloid Solution Colloid particles are much smaller than suspension particles. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other substance. Difference between suspension and colloid. Due to this size difference, colloid particles. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a. Difference Between Suspension And Colloid Solution.
From byjus.com
Suspensions & Colloids Difference Between Colloid & SuspensionByju's Difference Between Suspension And Colloid Solution The true solution is the homogenous mixture, while colloidal solution and suspension are the heterogeneous mixtures of two or more substances. The particles in a solution are much. Another difference between these three. Due to this size difference, colloid particles. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other. Difference Between Suspension And Colloid Solution.
From plantlet.org
Mixture Types Solution, Suspension, Colloids & Others Plantlet Difference Between Suspension And Colloid Solution The particles in a solution are much. Due to this size difference, colloid particles. Difference between suspension and colloid. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other substance. The main difference between colloid and suspension lies in the size of particles. The very first difference between the two.. Difference Between Suspension And Colloid Solution.
From www.youtube.com
Differences between True solutions, suspension & Colloid Part 12 Cbse Difference Between Suspension And Colloid Solution The main difference between colloid and suspension lies in the size of particles. Difference between suspension and colloid. Due to this size difference, colloid particles. Colloid particles are much smaller than suspension particles. Let’s explore some of the primary differences between colloids and suspensions. The solute particles in the true solution do not settle.the solute particles in the colloidal solution. Difference Between Suspension And Colloid Solution.
From loezijvab.blob.core.windows.net
Colloid Solution Definition at Gary Reese blog Difference Between Suspension And Colloid Solution The true solution is the homogenous mixture, while colloidal solution and suspension are the heterogeneous mixtures of two or more substances. The solute particles in the true solution do not settle.the solute particles in the colloidal solution can be made to settle by centrifugation.the solute. Colloid particles are much smaller than suspension particles. The very first difference between the two.. Difference Between Suspension And Colloid Solution.
From www.slideserve.com
PPT Suspensions and Colloids PowerPoint Presentation, free download Difference Between Suspension And Colloid Solution Another difference between these three. Difference between suspension and colloid. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other substance. The true solution is the homogenous mixture, while colloidal solution and suspension are the heterogeneous mixtures of two or more substances. The very first difference between the two. A. Difference Between Suspension And Colloid Solution.
From www.differencebetween.net
Difference Between Colloid and Suspension Difference Between Difference Between Suspension And Colloid Solution A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other substance. Let’s explore some of the primary differences between colloids and suspensions. Difference between suspension and colloid. The true solution is the homogenous mixture, while colloidal solution and suspension are the heterogeneous mixtures of two or more substances. The main. Difference Between Suspension And Colloid Solution.
From www.youtube.com
Colloids, Solutions & Suspensions YouTube Difference Between Suspension And Colloid Solution A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Let’s explore some of the primary differences between colloids and suspensions. Difference between suspension and colloid. Another difference between these three. The solute particles in the true solution do not settle.the solute particles in the colloidal solution. Difference Between Suspension And Colloid Solution.
From byjus.com
Suspensions (Chemistry) Definition, Properties, Examples with Videos Difference Between Suspension And Colloid Solution Colloid particles are much smaller than suspension particles. The solute particles in the true solution do not settle.the solute particles in the colloidal solution can be made to settle by centrifugation.the solute. Difference between suspension and colloid. Due to this size difference, colloid particles. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically. Difference Between Suspension And Colloid Solution.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of Difference Between Suspension And Colloid Solution The solute particles in the true solution do not settle.the solute particles in the colloidal solution can be made to settle by centrifugation.the solute. The main difference between colloid and suspension lies in the size of particles. Another difference between these three. Due to this size difference, colloid particles. Difference between suspension and colloid. The very first difference between the. Difference Between Suspension And Colloid Solution.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Difference Between Suspension And Colloid Solution A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. The very first difference between the two. The solute particles in the true solution do not settle.the solute particles in the colloidal solution can be made to settle by centrifugation.the solute. Another difference between these three. Let’s. Difference Between Suspension And Colloid Solution.
From quizizz.com
Solution, Colloid and Suspension Science Quizizz Difference Between Suspension And Colloid Solution Another difference between these three. The particles in a solution are much. The very first difference between the two. Let’s explore some of the primary differences between colloids and suspensions. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other substance. The solute particles in the true solution do not. Difference Between Suspension And Colloid Solution.
From brainly.in
what is the different between solution suspension and colloid Brainly.in Difference Between Suspension And Colloid Solution Let’s explore some of the primary differences between colloids and suspensions. A colloid is a heterogeneous mixture in which the dispersed particles are intermediate in size between those of a solution and a suspension. Another difference between these three. The particles in a solution are much. Due to this size difference, colloid particles. The solute particles in the true solution. Difference Between Suspension And Colloid Solution.
From www.slideserve.com
PPT Solution, Suspensions and Colloids PowerPoint Presentation, free Difference Between Suspension And Colloid Solution The main difference between colloid and suspension lies in the size of particles. Due to this size difference, colloid particles. The solute particles in the true solution do not settle.the solute particles in the colloidal solution can be made to settle by centrifugation.the solute. Colloid particles are much smaller than suspension particles. The very first difference between the two. Let’s. Difference Between Suspension And Colloid Solution.