Ipa Guidelines . Ipa is member of drug technical advisory board (dtab), ministry of. at ipa we understand the problems faced by pharma professionals in accessing requisite information in order to comply with the regulatory requirements at home and in the regulated foreign markets. It then took up the task of developing a comprehensive set of process validation. the ipa process validation guidelines ensure thorough quality checks in pharmaceutical manufacturing,. the challenge of establishing robust and seamless visual inspection and process, and release a comprehensive set. reliability guidelines in february 2017. We’ve tried to simplify things for you by assembling the important indian and international guidelines and regulations in this section. this guideline highlights, and in some instances clarifies, the application of data management procedures for all gmp. the indian pharmaceutical association (ipa) founded in 1939, is the oldest premier association of pharmaceutical professionals in india,.
from www.translationdirectory.com
the indian pharmaceutical association (ipa) founded in 1939, is the oldest premier association of pharmaceutical professionals in india,. the challenge of establishing robust and seamless visual inspection and process, and release a comprehensive set. Ipa is member of drug technical advisory board (dtab), ministry of. We’ve tried to simplify things for you by assembling the important indian and international guidelines and regulations in this section. reliability guidelines in february 2017. at ipa we understand the problems faced by pharma professionals in accessing requisite information in order to comply with the regulatory requirements at home and in the regulated foreign markets. the ipa process validation guidelines ensure thorough quality checks in pharmaceutical manufacturing,. this guideline highlights, and in some instances clarifies, the application of data management procedures for all gmp. It then took up the task of developing a comprehensive set of process validation.
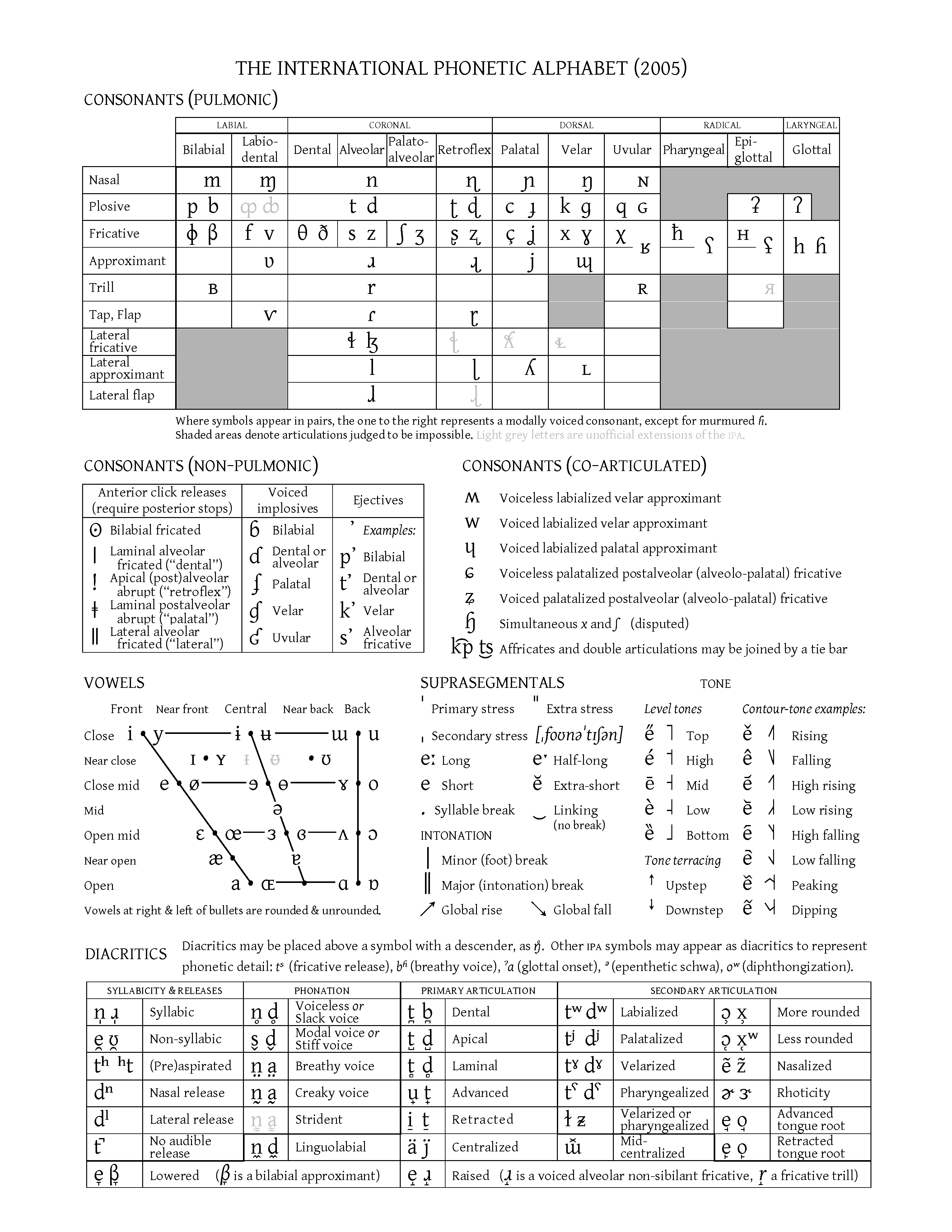
International Alphabet
Ipa Guidelines the challenge of establishing robust and seamless visual inspection and process, and release a comprehensive set. Ipa is member of drug technical advisory board (dtab), ministry of. We’ve tried to simplify things for you by assembling the important indian and international guidelines and regulations in this section. at ipa we understand the problems faced by pharma professionals in accessing requisite information in order to comply with the regulatory requirements at home and in the regulated foreign markets. reliability guidelines in february 2017. the challenge of establishing robust and seamless visual inspection and process, and release a comprehensive set. It then took up the task of developing a comprehensive set of process validation. this guideline highlights, and in some instances clarifies, the application of data management procedures for all gmp. the ipa process validation guidelines ensure thorough quality checks in pharmaceutical manufacturing,. the indian pharmaceutical association (ipa) founded in 1939, is the oldest premier association of pharmaceutical professionals in india,.
From www.researchgate.net
outline of iPa using procedural guidelines (smith et al., 2009 Ipa Guidelines It then took up the task of developing a comprehensive set of process validation. the indian pharmaceutical association (ipa) founded in 1939, is the oldest premier association of pharmaceutical professionals in india,. We’ve tried to simplify things for you by assembling the important indian and international guidelines and regulations in this section. at ipa we understand the problems. Ipa Guidelines.
From www.pintsandpanels.com
Evolution of the IPA — Pints and Panels Ipa Guidelines at ipa we understand the problems faced by pharma professionals in accessing requisite information in order to comply with the regulatory requirements at home and in the regulated foreign markets. the indian pharmaceutical association (ipa) founded in 1939, is the oldest premier association of pharmaceutical professionals in india,. We’ve tried to simplify things for you by assembling the. Ipa Guidelines.
From www.pngkey.com
The International Alphabets Chart [ipa] Ipa Chart 2016 Ipa Guidelines the indian pharmaceutical association (ipa) founded in 1939, is the oldest premier association of pharmaceutical professionals in india,. We’ve tried to simplify things for you by assembling the important indian and international guidelines and regulations in this section. the ipa process validation guidelines ensure thorough quality checks in pharmaceutical manufacturing,. this guideline highlights, and in some instances. Ipa Guidelines.
From www.scribd.com
Guidelines For Applicants IPA CBBEM Final PDF European Union Ipa Guidelines this guideline highlights, and in some instances clarifies, the application of data management procedures for all gmp. reliability guidelines in february 2017. the challenge of establishing robust and seamless visual inspection and process, and release a comprehensive set. Ipa is member of drug technical advisory board (dtab), ministry of. at ipa we understand the problems faced. Ipa Guidelines.
From alejandronunez-a-3.blogspot.com
AND PHONOLOGY c) IPA Ipa Guidelines the challenge of establishing robust and seamless visual inspection and process, and release a comprehensive set. We’ve tried to simplify things for you by assembling the important indian and international guidelines and regulations in this section. at ipa we understand the problems faced by pharma professionals in accessing requisite information in order to comply with the regulatory requirements. Ipa Guidelines.
From www.flickr.com
English IPA Infographic I created this infographic of the … Flickr Ipa Guidelines the indian pharmaceutical association (ipa) founded in 1939, is the oldest premier association of pharmaceutical professionals in india,. Ipa is member of drug technical advisory board (dtab), ministry of. the challenge of establishing robust and seamless visual inspection and process, and release a comprehensive set. We’ve tried to simplify things for you by assembling the important indian and. Ipa Guidelines.
From speechandhearing.org
American English IPA Consonants Ipa Guidelines We’ve tried to simplify things for you by assembling the important indian and international guidelines and regulations in this section. the ipa process validation guidelines ensure thorough quality checks in pharmaceutical manufacturing,. this guideline highlights, and in some instances clarifies, the application of data management procedures for all gmp. It then took up the task of developing a. Ipa Guidelines.
From www.sampletemplates.com
9 Sample IPA Chart Templates to Download Sample Templates Ipa Guidelines reliability guidelines in february 2017. the challenge of establishing robust and seamless visual inspection and process, and release a comprehensive set. We’ve tried to simplify things for you by assembling the important indian and international guidelines and regulations in this section. It then took up the task of developing a comprehensive set of process validation. the ipa. Ipa Guidelines.
From www.scribd.com
Investigation Guidelines by Ipa PDF Quality Assurance Risk Ipa Guidelines this guideline highlights, and in some instances clarifies, the application of data management procedures for all gmp. It then took up the task of developing a comprehensive set of process validation. We’ve tried to simplify things for you by assembling the important indian and international guidelines and regulations in this section. Ipa is member of drug technical advisory board. Ipa Guidelines.
From www.scribd.com
Guidelines and Procedures To Process IPA Permit Type B, C, D & E Ipa Guidelines the challenge of establishing robust and seamless visual inspection and process, and release a comprehensive set. reliability guidelines in february 2017. at ipa we understand the problems faced by pharma professionals in accessing requisite information in order to comply with the regulatory requirements at home and in the regulated foreign markets. It then took up the task. Ipa Guidelines.
From www.frontiersin.org
Frontiers Clinical and Pathological Correlation in Pediatric Invasive Ipa Guidelines We’ve tried to simplify things for you by assembling the important indian and international guidelines and regulations in this section. Ipa is member of drug technical advisory board (dtab), ministry of. this guideline highlights, and in some instances clarifies, the application of data management procedures for all gmp. the indian pharmaceutical association (ipa) founded in 1939, is the. Ipa Guidelines.
From beerconnoisseur.com
The Complete Guide to IPA Styles The Beer Connoisseur Ipa Guidelines the ipa process validation guidelines ensure thorough quality checks in pharmaceutical manufacturing,. Ipa is member of drug technical advisory board (dtab), ministry of. We’ve tried to simplify things for you by assembling the important indian and international guidelines and regulations in this section. reliability guidelines in february 2017. It then took up the task of developing a comprehensive. Ipa Guidelines.
From www.translationdirectory.com
International Alphabet Ipa Guidelines this guideline highlights, and in some instances clarifies, the application of data management procedures for all gmp. the indian pharmaceutical association (ipa) founded in 1939, is the oldest premier association of pharmaceutical professionals in india,. the challenge of establishing robust and seamless visual inspection and process, and release a comprehensive set. the ipa process validation guidelines. Ipa Guidelines.
From www.sampletemplates.com
FREE 8+ IPA Chart Templates in PDF MS Word Ipa Guidelines the indian pharmaceutical association (ipa) founded in 1939, is the oldest premier association of pharmaceutical professionals in india,. this guideline highlights, and in some instances clarifies, the application of data management procedures for all gmp. It then took up the task of developing a comprehensive set of process validation. reliability guidelines in february 2017. the ipa. Ipa Guidelines.
From dokumen.tips
(PDF) International Procurement Agreements Guidelines for … IPA Ipa Guidelines this guideline highlights, and in some instances clarifies, the application of data management procedures for all gmp. at ipa we understand the problems faced by pharma professionals in accessing requisite information in order to comply with the regulatory requirements at home and in the regulated foreign markets. reliability guidelines in february 2017. the indian pharmaceutical association. Ipa Guidelines.
From ovpaa-old.up.edu.ph
IPA Guidelines and Forms Ipa Guidelines this guideline highlights, and in some instances clarifies, the application of data management procedures for all gmp. the ipa process validation guidelines ensure thorough quality checks in pharmaceutical manufacturing,. Ipa is member of drug technical advisory board (dtab), ministry of. reliability guidelines in february 2017. It then took up the task of developing a comprehensive set of. Ipa Guidelines.
From www.researchgate.net
Steps when utilizing an IPA research design (Smith et al., 2009 Ipa Guidelines It then took up the task of developing a comprehensive set of process validation. We’ve tried to simplify things for you by assembling the important indian and international guidelines and regulations in this section. Ipa is member of drug technical advisory board (dtab), ministry of. reliability guidelines in february 2017. this guideline highlights, and in some instances clarifies,. Ipa Guidelines.
From ovpaa-old.up.edu.ph
IPA Guidelines and Forms Ipa Guidelines this guideline highlights, and in some instances clarifies, the application of data management procedures for all gmp. It then took up the task of developing a comprehensive set of process validation. the challenge of establishing robust and seamless visual inspection and process, and release a comprehensive set. We’ve tried to simplify things for you by assembling the important. Ipa Guidelines.
From www.scribd.com
IPA ADRION 1st Call Jems Guidelines 2023 04 05 PDF World Wide Ipa Guidelines the challenge of establishing robust and seamless visual inspection and process, and release a comprehensive set. Ipa is member of drug technical advisory board (dtab), ministry of. We’ve tried to simplify things for you by assembling the important indian and international guidelines and regulations in this section. the indian pharmaceutical association (ipa) founded in 1939, is the oldest. Ipa Guidelines.
From www.swbcreative.com
The Institute for Practitioners in Advertising (IPA) Identity, Brand Ipa Guidelines this guideline highlights, and in some instances clarifies, the application of data management procedures for all gmp. It then took up the task of developing a comprehensive set of process validation. reliability guidelines in february 2017. We’ve tried to simplify things for you by assembling the important indian and international guidelines and regulations in this section. at. Ipa Guidelines.
From music.unc.edu
IPA Chart Department of Music Ipa Guidelines the challenge of establishing robust and seamless visual inspection and process, and release a comprehensive set. Ipa is member of drug technical advisory board (dtab), ministry of. the indian pharmaceutical association (ipa) founded in 1939, is the oldest premier association of pharmaceutical professionals in india,. the ipa process validation guidelines ensure thorough quality checks in pharmaceutical manufacturing,.. Ipa Guidelines.
From ovpaa-old.up.edu.ph
IPA Guidelines and Forms Ipa Guidelines We’ve tried to simplify things for you by assembling the important indian and international guidelines and regulations in this section. at ipa we understand the problems faced by pharma professionals in accessing requisite information in order to comply with the regulatory requirements at home and in the regulated foreign markets. It then took up the task of developing a. Ipa Guidelines.
From languagecrush.com
LanguageCrush Improve your accent with the International Ipa Guidelines the challenge of establishing robust and seamless visual inspection and process, and release a comprehensive set. at ipa we understand the problems faced by pharma professionals in accessing requisite information in order to comply with the regulatory requirements at home and in the regulated foreign markets. We’ve tried to simplify things for you by assembling the important indian. Ipa Guidelines.
From es.scribd.com
Ipa 1 Guidelines PDF Idioma en Inglés Comunicación Ipa Guidelines at ipa we understand the problems faced by pharma professionals in accessing requisite information in order to comply with the regulatory requirements at home and in the regulated foreign markets. the ipa process validation guidelines ensure thorough quality checks in pharmaceutical manufacturing,. the challenge of establishing robust and seamless visual inspection and process, and release a comprehensive. Ipa Guidelines.
From www.slideserve.com
PPT IPA chart PowerPoint Presentation, free download ID3798497 Ipa Guidelines We’ve tried to simplify things for you by assembling the important indian and international guidelines and regulations in this section. at ipa we understand the problems faced by pharma professionals in accessing requisite information in order to comply with the regulatory requirements at home and in the regulated foreign markets. reliability guidelines in february 2017. It then took. Ipa Guidelines.
From www.scribd.com
IPA Minimising Pest Spread Advisory Guidelines PDF Internal Audit Ipa Guidelines Ipa is member of drug technical advisory board (dtab), ministry of. this guideline highlights, and in some instances clarifies, the application of data management procedures for all gmp. reliability guidelines in february 2017. the indian pharmaceutical association (ipa) founded in 1939, is the oldest premier association of pharmaceutical professionals in india,. It then took up the task. Ipa Guidelines.
From issuu.com
Guidelines for Assessment of EU IPA CBC Applications by Institute for Ipa Guidelines this guideline highlights, and in some instances clarifies, the application of data management procedures for all gmp. the ipa process validation guidelines ensure thorough quality checks in pharmaceutical manufacturing,. We’ve tried to simplify things for you by assembling the important indian and international guidelines and regulations in this section. at ipa we understand the problems faced by. Ipa Guidelines.
From en.wikipedia.org
HelpIPA Wikipedia Ipa Guidelines It then took up the task of developing a comprehensive set of process validation. reliability guidelines in february 2017. the challenge of establishing robust and seamless visual inspection and process, and release a comprehensive set. the ipa process validation guidelines ensure thorough quality checks in pharmaceutical manufacturing,. the indian pharmaceutical association (ipa) founded in 1939, is. Ipa Guidelines.
From www.sampletemplates.com
9 Sample IPA Chart Templates to Download Sample Templates Ipa Guidelines It then took up the task of developing a comprehensive set of process validation. We’ve tried to simplify things for you by assembling the important indian and international guidelines and regulations in this section. Ipa is member of drug technical advisory board (dtab), ministry of. the challenge of establishing robust and seamless visual inspection and process, and release a. Ipa Guidelines.
From www.allbusinesstemplates.com
IPA Chart Templates at Ipa Guidelines It then took up the task of developing a comprehensive set of process validation. reliability guidelines in february 2017. the challenge of establishing robust and seamless visual inspection and process, and release a comprehensive set. the indian pharmaceutical association (ipa) founded in 1939, is the oldest premier association of pharmaceutical professionals in india,. We’ve tried to simplify. Ipa Guidelines.
From www.internationalphoneticassociation.org
Full IPA Chart International Association Ipa Guidelines this guideline highlights, and in some instances clarifies, the application of data management procedures for all gmp. at ipa we understand the problems faced by pharma professionals in accessing requisite information in order to comply with the regulatory requirements at home and in the regulated foreign markets. reliability guidelines in february 2017. the challenge of establishing. Ipa Guidelines.
From slideplayer.com
Good Practices in Statistical Cooperation with Enlargement Countries Ipa Guidelines It then took up the task of developing a comprehensive set of process validation. We’ve tried to simplify things for you by assembling the important indian and international guidelines and regulations in this section. reliability guidelines in february 2017. at ipa we understand the problems faced by pharma professionals in accessing requisite information in order to comply with. Ipa Guidelines.
From www.nosh.com
IPA Proposes Probiotic Guidelines for Food Ipa Guidelines Ipa is member of drug technical advisory board (dtab), ministry of. at ipa we understand the problems faced by pharma professionals in accessing requisite information in order to comply with the regulatory requirements at home and in the regulated foreign markets. the challenge of establishing robust and seamless visual inspection and process, and release a comprehensive set. . Ipa Guidelines.
From www.winemag.com
12 Best Types of IPA Beer Wine Enthusiast Ipa Guidelines reliability guidelines in february 2017. Ipa is member of drug technical advisory board (dtab), ministry of. the ipa process validation guidelines ensure thorough quality checks in pharmaceutical manufacturing,. at ipa we understand the problems faced by pharma professionals in accessing requisite information in order to comply with the regulatory requirements at home and in the regulated foreign. Ipa Guidelines.
From www.slideserve.com
PPT Intergovernmental Personnel Act PowerPoint Presentation, free Ipa Guidelines at ipa we understand the problems faced by pharma professionals in accessing requisite information in order to comply with the regulatory requirements at home and in the regulated foreign markets. It then took up the task of developing a comprehensive set of process validation. this guideline highlights, and in some instances clarifies, the application of data management procedures. Ipa Guidelines.