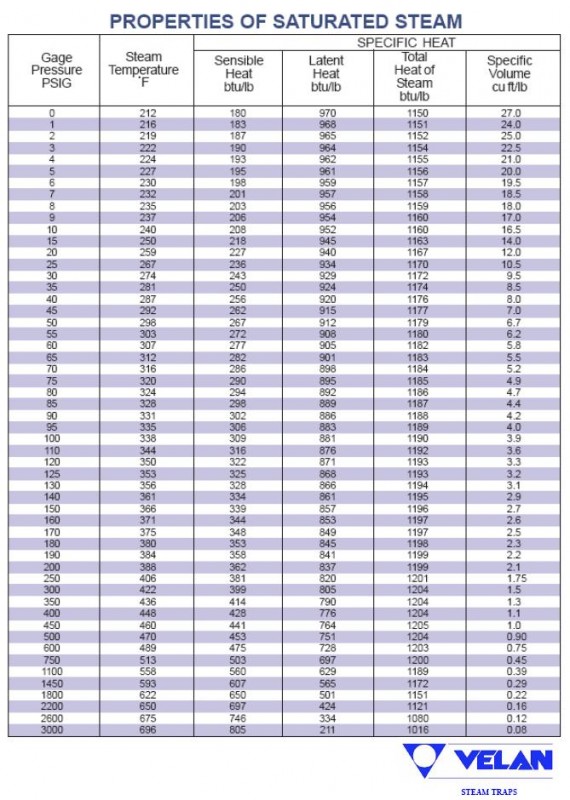
Does Steam Temperature Increase With Pressure . the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). For example, steam at 620 psig. Steam should reach the point of use at the required pressure and provide the desired. correct pressure and temp erature of steam. Calculate propierties of wet, saturated and superheated steam, steam quality and. by superheating steam, we can add enthalpy to steam without raising the pressure of the steam. steam with a temperature equal to the boiling point at that pressure is known as dry saturated steam. the relationship between the saturation temperature and the pressure is known as the steam saturation curve (see image below). steam tables online calculator, completely free!
from keplarllp.com
correct pressure and temp erature of steam. the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). by superheating steam, we can add enthalpy to steam without raising the pressure of the steam. Steam should reach the point of use at the required pressure and provide the desired. steam with a temperature equal to the boiling point at that pressure is known as dry saturated steam. steam tables online calculator, completely free! Calculate propierties of wet, saturated and superheated steam, steam quality and. the relationship between the saturation temperature and the pressure is known as the steam saturation curve (see image below). For example, steam at 620 psig.
😂 Steam pressure and temperature relationship. Steam Heating System
Does Steam Temperature Increase With Pressure steam tables online calculator, completely free! Calculate propierties of wet, saturated and superheated steam, steam quality and. steam tables online calculator, completely free! correct pressure and temp erature of steam. steam with a temperature equal to the boiling point at that pressure is known as dry saturated steam. Steam should reach the point of use at the required pressure and provide the desired. the relationship between the saturation temperature and the pressure is known as the steam saturation curve (see image below). For example, steam at 620 psig. by superheating steam, we can add enthalpy to steam without raising the pressure of the steam. the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\).
From instrumentationtools.com
Steam Pressure Control Power Plant Training Resources Does Steam Temperature Increase With Pressure the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). by superheating steam, we can add enthalpy to steam without raising the pressure of the steam. correct pressure and temp erature of steam. Calculate propierties of wet, saturated and superheated steam, steam quality and. For example, steam at. Does Steam Temperature Increase With Pressure.
From www.researchgate.net
Effects of reduced steam pressure and increased superheating Download Does Steam Temperature Increase With Pressure correct pressure and temp erature of steam. Steam should reach the point of use at the required pressure and provide the desired. steam with a temperature equal to the boiling point at that pressure is known as dry saturated steam. the relationship between the saturation temperature and the pressure is known as the steam saturation curve (see. Does Steam Temperature Increase With Pressure.
From www.410achiller.com
Temperature Pressure Chart R134A R407C R404A R410A Does Steam Temperature Increase With Pressure Steam should reach the point of use at the required pressure and provide the desired. For example, steam at 620 psig. steam tables online calculator, completely free! correct pressure and temp erature of steam. steam with a temperature equal to the boiling point at that pressure is known as dry saturated steam. by superheating steam, we. Does Steam Temperature Increase With Pressure.
From www.nuclear-power.com
Rankine Cycle Steam Turbine Cycle Characteristics Does Steam Temperature Increase With Pressure Calculate propierties of wet, saturated and superheated steam, steam quality and. steam tables online calculator, completely free! Steam should reach the point of use at the required pressure and provide the desired. by superheating steam, we can add enthalpy to steam without raising the pressure of the steam. the relationships among the volume of a gas and. Does Steam Temperature Increase With Pressure.
From www.esmagazine.com
Designing A Steam Systems For A Brewery 20160201 Engineered Does Steam Temperature Increase With Pressure For example, steam at 620 psig. steam with a temperature equal to the boiling point at that pressure is known as dry saturated steam. steam tables online calculator, completely free! the relationship between the saturation temperature and the pressure is known as the steam saturation curve (see image below). the relationships among the volume of a. Does Steam Temperature Increase With Pressure.
From www.hkdivedi.com
TEMPERATURE ENTROPY DIAGRAM FOR WATER ENGINEERING APPLICATIONS Does Steam Temperature Increase With Pressure by superheating steam, we can add enthalpy to steam without raising the pressure of the steam. the relationship between the saturation temperature and the pressure is known as the steam saturation curve (see image below). the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). correct pressure. Does Steam Temperature Increase With Pressure.
From www.researchgate.net
Sensitivity effects of steam pressure, steam temperature and feedwater Does Steam Temperature Increase With Pressure Steam should reach the point of use at the required pressure and provide the desired. the relationship between the saturation temperature and the pressure is known as the steam saturation curve (see image below). steam tables online calculator, completely free! Calculate propierties of wet, saturated and superheated steam, steam quality and. by superheating steam, we can add. Does Steam Temperature Increase With Pressure.
From mungfali.com
Steam Pressure Chart Does Steam Temperature Increase With Pressure Steam should reach the point of use at the required pressure and provide the desired. the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). For example, steam at 620 psig. correct pressure and temp erature of steam. steam tables online calculator, completely free! Calculate propierties of wet,. Does Steam Temperature Increase With Pressure.
From www.formsbirds.com
Steam Pressure Temperature Chart Free Download Does Steam Temperature Increase With Pressure steam with a temperature equal to the boiling point at that pressure is known as dry saturated steam. Steam should reach the point of use at the required pressure and provide the desired. For example, steam at 620 psig. the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\).. Does Steam Temperature Increase With Pressure.
From heat-transfer-thermodynamics.blogspot.com
Heat Transfer and Applied Thermodynamics Condensation from the Atmosphere Does Steam Temperature Increase With Pressure Steam should reach the point of use at the required pressure and provide the desired. For example, steam at 620 psig. correct pressure and temp erature of steam. the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). by superheating steam, we can add enthalpy to steam without. Does Steam Temperature Increase With Pressure.
From schematicrogueelephantn6.z14.web.core.windows.net
Two Pipe Steam System Does Steam Temperature Increase With Pressure the relationship between the saturation temperature and the pressure is known as the steam saturation curve (see image below). by superheating steam, we can add enthalpy to steam without raising the pressure of the steam. Steam should reach the point of use at the required pressure and provide the desired. steam tables online calculator, completely free! Calculate. Does Steam Temperature Increase With Pressure.
From hvacrschool.com
Saturation and the PressureTemperature Relationship HVAC School Does Steam Temperature Increase With Pressure Calculate propierties of wet, saturated and superheated steam, steam quality and. correct pressure and temp erature of steam. For example, steam at 620 psig. by superheating steam, we can add enthalpy to steam without raising the pressure of the steam. the relationship between the saturation temperature and the pressure is known as the steam saturation curve (see. Does Steam Temperature Increase With Pressure.
From recipepes.com
steam temperature chart Does Steam Temperature Increase With Pressure Steam should reach the point of use at the required pressure and provide the desired. Calculate propierties of wet, saturated and superheated steam, steam quality and. steam tables online calculator, completely free! steam with a temperature equal to the boiling point at that pressure is known as dry saturated steam. For example, steam at 620 psig. the. Does Steam Temperature Increase With Pressure.
From recipepes.com
steam temperature chart Does Steam Temperature Increase With Pressure Calculate propierties of wet, saturated and superheated steam, steam quality and. Steam should reach the point of use at the required pressure and provide the desired. correct pressure and temp erature of steam. steam tables online calculator, completely free! For example, steam at 620 psig. the relationship between the saturation temperature and the pressure is known as. Does Steam Temperature Increase With Pressure.
From www.quizbroz.com
Solved Question The pressure in a steam boiler is given to be 75 kgf Does Steam Temperature Increase With Pressure Steam should reach the point of use at the required pressure and provide the desired. correct pressure and temp erature of steam. steam with a temperature equal to the boiling point at that pressure is known as dry saturated steam. by superheating steam, we can add enthalpy to steam without raising the pressure of the steam. Calculate. Does Steam Temperature Increase With Pressure.
From elliot-has-arias.blogspot.com
Compressible Fluid Flow Equations ElliothasArias Does Steam Temperature Increase With Pressure Steam should reach the point of use at the required pressure and provide the desired. the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). by superheating steam, we can add enthalpy to steam without raising the pressure of the steam. the relationship between the saturation temperature and. Does Steam Temperature Increase With Pressure.
From socratic.org
What is the relation between critical temperature and boiling point or Does Steam Temperature Increase With Pressure the relationship between the saturation temperature and the pressure is known as the steam saturation curve (see image below). correct pressure and temp erature of steam. the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Calculate propierties of wet, saturated and superheated steam, steam quality and. For. Does Steam Temperature Increase With Pressure.
From mavink.com
Pressure And Temperature Graph Does Steam Temperature Increase With Pressure by superheating steam, we can add enthalpy to steam without raising the pressure of the steam. Calculate propierties of wet, saturated and superheated steam, steam quality and. Steam should reach the point of use at the required pressure and provide the desired. steam with a temperature equal to the boiling point at that pressure is known as dry. Does Steam Temperature Increase With Pressure.
From www.numerade.com
SOLVED A Steam Power Plant operates on the Ideal Reheat Rankine Cycle Does Steam Temperature Increase With Pressure Calculate propierties of wet, saturated and superheated steam, steam quality and. For example, steam at 620 psig. the relationship between the saturation temperature and the pressure is known as the steam saturation curve (see image below). correct pressure and temp erature of steam. the relationships among the volume of a gas and its pressure, temperature, and amount. Does Steam Temperature Increase With Pressure.
From kenkidryer.com
Saturation temperature (boiling point) KENKI DRYER Does Steam Temperature Increase With Pressure steam with a temperature equal to the boiling point at that pressure is known as dry saturated steam. Calculate propierties of wet, saturated and superheated steam, steam quality and. the relationship between the saturation temperature and the pressure is known as the steam saturation curve (see image below). For example, steam at 620 psig. by superheating steam,. Does Steam Temperature Increase With Pressure.
From www.researchgate.net
21. Relationships between steam pressure, temperature and specific Does Steam Temperature Increase With Pressure the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). correct pressure and temp erature of steam. steam tables online calculator, completely free! the relationship between the saturation temperature and the pressure is known as the steam saturation curve (see image below). Steam should reach the point. Does Steam Temperature Increase With Pressure.
From www.boiler-planning.com
Flue gas temperature or flue gas loss Bosch Steam boiler planning Does Steam Temperature Increase With Pressure correct pressure and temp erature of steam. the relationship between the saturation temperature and the pressure is known as the steam saturation curve (see image below). steam with a temperature equal to the boiling point at that pressure is known as dry saturated steam. Steam should reach the point of use at the required pressure and provide. Does Steam Temperature Increase With Pressure.
From www.myxxgirl.com
Pressure Temperature Chart My XXX Hot Girl Does Steam Temperature Increase With Pressure Calculate propierties of wet, saturated and superheated steam, steam quality and. by superheating steam, we can add enthalpy to steam without raising the pressure of the steam. the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). steam with a temperature equal to the boiling point at that. Does Steam Temperature Increase With Pressure.
From www.formsbirds.com
Pressure Temperature Chart 6 Free Templates in PDF, Word, Excel Download Does Steam Temperature Increase With Pressure the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). the relationship between the saturation temperature and the pressure is known as the steam saturation curve (see image below). For example, steam at 620 psig. correct pressure and temp erature of steam. steam tables online calculator, completely. Does Steam Temperature Increase With Pressure.
From dw-inductionheater.com
Applications and Advantages of Induction Heating Steam Boiler Does Steam Temperature Increase With Pressure the relationship between the saturation temperature and the pressure is known as the steam saturation curve (see image below). correct pressure and temp erature of steam. steam tables online calculator, completely free! by superheating steam, we can add enthalpy to steam without raising the pressure of the steam. steam with a temperature equal to the. Does Steam Temperature Increase With Pressure.
From www.scribd.com
Saturated Steam Temperature Table Steam Power Nature Does Steam Temperature Increase With Pressure the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). by superheating steam, we can add enthalpy to steam without raising the pressure of the steam. Calculate propierties of wet, saturated and superheated steam, steam quality and. Steam should reach the point of use at the required pressure and. Does Steam Temperature Increase With Pressure.
From www.formsbank.com
TemperaturePressure Equivalent Chart Of Saturated Steam printable pdf Does Steam Temperature Increase With Pressure steam with a temperature equal to the boiling point at that pressure is known as dry saturated steam. Steam should reach the point of use at the required pressure and provide the desired. the relationship between the saturation temperature and the pressure is known as the steam saturation curve (see image below). For example, steam at 620 psig.. Does Steam Temperature Increase With Pressure.
From keplarllp.com
😂 Steam pressure and temperature relationship. Steam Heating System Does Steam Temperature Increase With Pressure steam with a temperature equal to the boiling point at that pressure is known as dry saturated steam. the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). Steam should reach the point of use at the required pressure and provide the desired. Calculate propierties of wet, saturated and. Does Steam Temperature Increase With Pressure.
From www.valvesonline.com.au
Steam Tables Pressure vs Temperature Does Steam Temperature Increase With Pressure steam tables online calculator, completely free! correct pressure and temp erature of steam. Steam should reach the point of use at the required pressure and provide the desired. Calculate propierties of wet, saturated and superheated steam, steam quality and. the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure. Does Steam Temperature Increase With Pressure.
From www.researchgate.net
Saturation pressure as a function of the saturation temperature for Does Steam Temperature Increase With Pressure correct pressure and temp erature of steam. Calculate propierties of wet, saturated and superheated steam, steam quality and. steam tables online calculator, completely free! the relationship between the saturation temperature and the pressure is known as the steam saturation curve (see image below). steam with a temperature equal to the boiling point at that pressure is. Does Steam Temperature Increase With Pressure.
From www.researchgate.net
Variation saturation temperature against steam pressure Download Does Steam Temperature Increase With Pressure by superheating steam, we can add enthalpy to steam without raising the pressure of the steam. steam tables online calculator, completely free! steam with a temperature equal to the boiling point at that pressure is known as dry saturated steam. For example, steam at 620 psig. Steam should reach the point of use at the required pressure. Does Steam Temperature Increase With Pressure.
From blog.opticontrols.com
Steam Temperature Control Control Notes Does Steam Temperature Increase With Pressure Steam should reach the point of use at the required pressure and provide the desired. correct pressure and temp erature of steam. For example, steam at 620 psig. the relationship between the saturation temperature and the pressure is known as the steam saturation curve (see image below). steam tables online calculator, completely free! the relationships among. Does Steam Temperature Increase With Pressure.
From www.boiler-planning.com
Boiling pressure and temperature Bosch Steam boiler planning Does Steam Temperature Increase With Pressure Steam should reach the point of use at the required pressure and provide the desired. steam with a temperature equal to the boiling point at that pressure is known as dry saturated steam. correct pressure and temp erature of steam. the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in. Does Steam Temperature Increase With Pressure.
From www.atlascopco.com
Verzadigde versus onverzadigde stoom Atlas Copco Netherlands Does Steam Temperature Increase With Pressure For example, steam at 620 psig. Steam should reach the point of use at the required pressure and provide the desired. by superheating steam, we can add enthalpy to steam without raising the pressure of the steam. correct pressure and temp erature of steam. steam tables online calculator, completely free! Calculate propierties of wet, saturated and superheated. Does Steam Temperature Increase With Pressure.
From keplarllp.com
😂 Steam pressure and temperature relationship. Steam Heating System Does Steam Temperature Increase With Pressure the relationship between the saturation temperature and the pressure is known as the steam saturation curve (see image below). correct pressure and temp erature of steam. steam tables online calculator, completely free! the relationships among the volume of a gas and its pressure, temperature, and amount are summarized in figure \(\pageindex{5}\). For example, steam at 620. Does Steam Temperature Increase With Pressure.