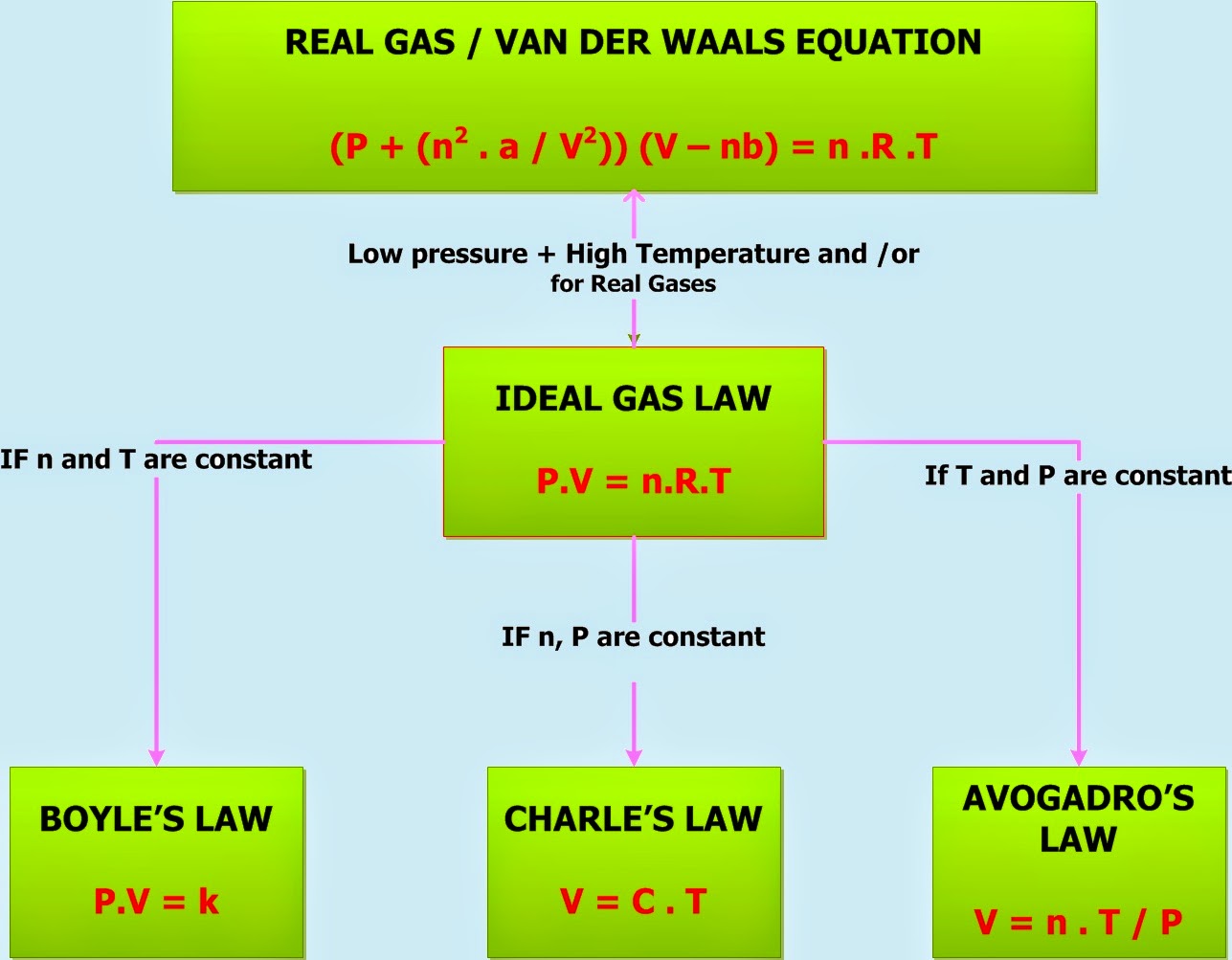
Gas Law Equation Sheet . the ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. the ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? Specifying any four of these terms will permit use of the ideal gas law to calculate the fifth term as demonstrated in the following example exercises. Calculate the decrease in temperature when 2.00 l at 20.0 °c is compressed to. the ideal gas equation contains five terms, the gas constant r and the variable properties p, v, n, and t. The rate of effusion/diffusion of two. at low pressure (less than 1 atmosphere) and high temperature (greater than 0°c), most gases obey the ideal gas equation: Use your knowledge of the ideal and combined gas laws to solve the following problems. Assume that the lungs are at 1.00. the ideal and combined gas laws pv = nrt or p1v1 = p2v2.
from learningmagicharlow.z13.web.core.windows.net
at low pressure (less than 1 atmosphere) and high temperature (greater than 0°c), most gases obey the ideal gas equation: Use your knowledge of the ideal and combined gas laws to solve the following problems. The rate of effusion/diffusion of two. the ideal and combined gas laws pv = nrt or p1v1 = p2v2. the ideal gas equation contains five terms, the gas constant r and the variable properties p, v, n, and t. the ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? Calculate the decrease in temperature when 2.00 l at 20.0 °c is compressed to. Specifying any four of these terms will permit use of the ideal gas law to calculate the fifth term as demonstrated in the following example exercises. the ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”.
Formulas For Gas Laws
Gas Law Equation Sheet the ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. The rate of effusion/diffusion of two. at low pressure (less than 1 atmosphere) and high temperature (greater than 0°c), most gases obey the ideal gas equation: the ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. the ideal gas equation contains five terms, the gas constant r and the variable properties p, v, n, and t. Assume that the lungs are at 1.00. the ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. Specifying any four of these terms will permit use of the ideal gas law to calculate the fifth term as demonstrated in the following example exercises. Calculate the decrease in temperature when 2.00 l at 20.0 °c is compressed to. the ideal and combined gas laws pv = nrt or p1v1 = p2v2. Use your knowledge of the ideal and combined gas laws to solve the following problems. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l?
From byjus.com
Ideal Gas Law Equation Compressibility Of Natural Gas Chemistry Gas Law Equation Sheet at low pressure (less than 1 atmosphere) and high temperature (greater than 0°c), most gases obey the ideal gas equation: Specifying any four of these terms will permit use of the ideal gas law to calculate the fifth term as demonstrated in the following example exercises. the ideal gas law relates the pressure, temperature, volume, and mass of. Gas Law Equation Sheet.
From mathformula5.netlify.app
What Is The Mathematical Formula Of Boyles Law Complete Guide Gas Law Equation Sheet Calculate the decrease in temperature when 2.00 l at 20.0 °c is compressed to. The rate of effusion/diffusion of two. the ideal and combined gas laws pv = nrt or p1v1 = p2v2. the ideal gas equation contains five terms, the gas constant r and the variable properties p, v, n, and t. How many moles of gas. Gas Law Equation Sheet.
From www.slideserve.com
PPT The Ideal Gas Equation PowerPoint Presentation, free download Gas Law Equation Sheet Assume that the lungs are at 1.00. at low pressure (less than 1 atmosphere) and high temperature (greater than 0°c), most gases obey the ideal gas equation: The rate of effusion/diffusion of two. the ideal and combined gas laws pv = nrt or p1v1 = p2v2. Use your knowledge of the ideal and combined gas laws to solve. Gas Law Equation Sheet.
From scienceducatio.weebly.com
Gas Laws Environmental Chemistry Gas Law Equation Sheet Use your knowledge of the ideal and combined gas laws to solve the following problems. Calculate the decrease in temperature when 2.00 l at 20.0 °c is compressed to. at low pressure (less than 1 atmosphere) and high temperature (greater than 0°c), most gases obey the ideal gas equation: the ideal gas equation contains five terms, the gas. Gas Law Equation Sheet.
From studiousguy.com
The Gas Laws Definition, Formula & Examples StudiousGuy Gas Law Equation Sheet The rate of effusion/diffusion of two. the ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. Specifying any four of these terms will permit use of the ideal gas law to calculate the fifth term as demonstrated in the following example exercises. the ideal gas equation contains five terms,. Gas Law Equation Sheet.
From www.slideserve.com
PPT The Ideal Gas Law PowerPoint Presentation, free download ID4354594 Gas Law Equation Sheet the ideal and combined gas laws pv = nrt or p1v1 = p2v2. the ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. the ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. Use your knowledge of the ideal. Gas Law Equation Sheet.
From www.slideserve.com
PPT IdealGas Equation PowerPoint Presentation, free download ID Gas Law Equation Sheet Use your knowledge of the ideal and combined gas laws to solve the following problems. the ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. Specifying any four of these terms will permit use of the ideal gas law to calculate the fifth term as demonstrated in the following example. Gas Law Equation Sheet.
From octavio-has-buchanan.blogspot.com
What Does Avogadros Law Say About a Gas at Stp OctaviohasBuchanan Gas Law Equation Sheet Calculate the decrease in temperature when 2.00 l at 20.0 °c is compressed to. Use your knowledge of the ideal and combined gas laws to solve the following problems. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? the ideal gas equation contains five terms, the gas constant. Gas Law Equation Sheet.
From www.youtube.com
Understanding the Gas Law Equations YouTube Gas Law Equation Sheet Specifying any four of these terms will permit use of the ideal gas law to calculate the fifth term as demonstrated in the following example exercises. Assume that the lungs are at 1.00. the ideal gas equation contains five terms, the gas constant r and the variable properties p, v, n, and t. at low pressure (less than. Gas Law Equation Sheet.
From printablefullglens.z13.web.core.windows.net
Formulas For Gas Laws Gas Law Equation Sheet Use your knowledge of the ideal and combined gas laws to solve the following problems. Specifying any four of these terms will permit use of the ideal gas law to calculate the fifth term as demonstrated in the following example exercises. at low pressure (less than 1 atmosphere) and high temperature (greater than 0°c), most gases obey the ideal. Gas Law Equation Sheet.
From www.expii.com
Combined Gas Law — Overview & Calculations Expii Gas Law Equation Sheet The rate of effusion/diffusion of two. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? at low pressure (less than 1 atmosphere) and high temperature (greater than 0°c), most gases obey the ideal gas equation: Assume that the lungs are at 1.00. Specifying any four of these terms. Gas Law Equation Sheet.
From studylib.net
GAS LAWS Equation Sheet Gas Law Equation Sheet Assume that the lungs are at 1.00. at low pressure (less than 1 atmosphere) and high temperature (greater than 0°c), most gases obey the ideal gas equation: Calculate the decrease in temperature when 2.00 l at 20.0 °c is compressed to. the ideal gas equation contains five terms, the gas constant r and the variable properties p, v,. Gas Law Equation Sheet.
From materiallibrarycochran.z4.web.core.windows.net
Gas Law Formulas Sheet Gas Law Equation Sheet Calculate the decrease in temperature when 2.00 l at 20.0 °c is compressed to. Assume that the lungs are at 1.00. The rate of effusion/diffusion of two. the ideal and combined gas laws pv = nrt or p1v1 = p2v2. Use your knowledge of the ideal and combined gas laws to solve the following problems. Specifying any four of. Gas Law Equation Sheet.
From learningmediaallan99.z13.web.core.windows.net
All Gas Law Formulas Gas Law Equation Sheet the ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. Calculate the decrease in temperature when 2.00 l at 20.0 °c is compressed to. the ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. The rate of effusion/diffusion of two.. Gas Law Equation Sheet.
From materialfullrobin.z13.web.core.windows.net
Gas Laws Equations Sheet Gas Law Equation Sheet the ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. Use your knowledge of the ideal and combined gas laws to solve the following problems. Calculate the decrease in temperature when 2.00 l at 20.0 °c is compressed to. the ideal and combined gas laws pv = nrt or. Gas Law Equation Sheet.
From worksheetfullkristi.z21.web.core.windows.net
Gas Laws Equations Sheet Gas Law Equation Sheet the ideal and combined gas laws pv = nrt or p1v1 = p2v2. the ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. at low pressure (less than 1 atmosphere) and high temperature (greater than 0°c), most gases obey the ideal gas equation: Assume that the lungs are. Gas Law Equation Sheet.
From mungfali.com
Gas Laws Cheat Sheet Gas Law Equation Sheet The rate of effusion/diffusion of two. Assume that the lungs are at 1.00. at low pressure (less than 1 atmosphere) and high temperature (greater than 0°c), most gases obey the ideal gas equation: How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? Calculate the decrease in temperature when. Gas Law Equation Sheet.
From worksheetfullsmokers.z22.web.core.windows.net
How To Calculate Gas Law Gas Law Equation Sheet Specifying any four of these terms will permit use of the ideal gas law to calculate the fifth term as demonstrated in the following example exercises. Assume that the lungs are at 1.00. the ideal and combined gas laws pv = nrt or p1v1 = p2v2. Calculate the decrease in temperature when 2.00 l at 20.0 °c is compressed. Gas Law Equation Sheet.
From www.britannica.com
Ideal gas law Definition, Formula, & Facts Britannica Gas Law Equation Sheet the ideal gas equation contains five terms, the gas constant r and the variable properties p, v, n, and t. the ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9. Gas Law Equation Sheet.
From www.youtube.com
Ideal Gas Equation How to Choose the Correct Gas Constant, R? With Gas Law Equation Sheet The rate of effusion/diffusion of two. Calculate the decrease in temperature when 2.00 l at 20.0 °c is compressed to. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? the ideal and combined gas laws pv = nrt or p1v1 = p2v2. Assume that the lungs are at. Gas Law Equation Sheet.
From mmerevise.co.uk
The Ideal Gas Equation MME Gas Law Equation Sheet the ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. Use your knowledge of the ideal and combined gas laws to solve the following problems. Specifying any four of these terms will permit use of the ideal gas law to calculate the fifth term as demonstrated in the following example. Gas Law Equation Sheet.
From printablelibmoits.z13.web.core.windows.net
Combined Gas Law Explained Gas Law Equation Sheet Calculate the decrease in temperature when 2.00 l at 20.0 °c is compressed to. The rate of effusion/diffusion of two. Use your knowledge of the ideal and combined gas laws to solve the following problems. the ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. the ideal gas law. Gas Law Equation Sheet.
From sciencenotes.org
Ideal Gas Law Formula and Examples Gas Law Equation Sheet Use your knowledge of the ideal and combined gas laws to solve the following problems. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? Specifying any four of these terms will permit use of the ideal gas law to calculate the fifth term as demonstrated in the following example. Gas Law Equation Sheet.
From www.expii.com
Ideal Gas Law — Overview & Calculations Expii Gas Law Equation Sheet Use your knowledge of the ideal and combined gas laws to solve the following problems. the ideal and combined gas laws pv = nrt or p1v1 = p2v2. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? Calculate the decrease in temperature when 2.00 l at 20.0 °c. Gas Law Equation Sheet.
From lessonlibraryungirths.z21.web.core.windows.net
Combined Gas Law Explained Gas Law Equation Sheet the ideal gas equation contains five terms, the gas constant r and the variable properties p, v, n, and t. the ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. Calculate the decrease in temperature when 2.00 l at 20.0 °c is compressed to. How many moles of gas. Gas Law Equation Sheet.
From sciencenotes.org
Combined Gas Law Definition, Formula, Examples Gas Law Equation Sheet How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? Calculate the decrease in temperature when 2.00 l at 20.0 °c is compressed to. Use your knowledge of the ideal and combined gas laws to solve the following problems. the ideal gas equation contains five terms, the gas constant. Gas Law Equation Sheet.
From www.youtube.com
Gas Law Formulas and Equations College Chemistry Study Guide YouTube Gas Law Equation Sheet Assume that the lungs are at 1.00. the ideal gas equation contains five terms, the gas constant r and the variable properties p, v, n, and t. Use your knowledge of the ideal and combined gas laws to solve the following problems. the ideal gas law relates the pressure, temperature, volume, and mass of a gas through the. Gas Law Equation Sheet.
From www.pinterest.de
This sheet gives the formulas for the Gas Laws. MA.912.HSAAPR.C Gas Law Equation Sheet How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? Specifying any four of these terms will permit use of the ideal gas law to calculate the fifth term as demonstrated in the following example exercises. Calculate the decrease in temperature when 2.00 l at 20.0 °c is compressed to.. Gas Law Equation Sheet.
From www.slideserve.com
PPT The General Gas Equation Combined Gas Law PowerPoint Presentation Gas Law Equation Sheet at low pressure (less than 1 atmosphere) and high temperature (greater than 0°c), most gases obey the ideal gas equation: Assume that the lungs are at 1.00. the ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. The rate of effusion/diffusion of two. the ideal gas law relates. Gas Law Equation Sheet.
From inspiritvr.com
Combined Gas Law Study Guide Inspirit Learning Inc Gas Law Equation Sheet Assume that the lungs are at 1.00. How many moles of gas (air) are in the lungs of an adult with a lung capacity of 3.9 l? at low pressure (less than 1 atmosphere) and high temperature (greater than 0°c), most gases obey the ideal gas equation: the ideal gas law relates the pressure, temperature, volume, and mass. Gas Law Equation Sheet.
From www.scribd.com
Exercises Sections 10.3, 10.4 The Gas Laws; The IdealGas Equation Gas Law Equation Sheet at low pressure (less than 1 atmosphere) and high temperature (greater than 0°c), most gases obey the ideal gas equation: the ideal and combined gas laws pv = nrt or p1v1 = p2v2. the ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. the ideal gas equation. Gas Law Equation Sheet.
From www.chem.fsu.edu
Gas Laws Gas Law Equation Sheet the ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. the ideal gas equation contains five terms, the gas constant r and the variable properties p, v, n, and t. at low pressure (less than 1 atmosphere) and high temperature (greater than 0°c), most gases obey the ideal. Gas Law Equation Sheet.
From www.expii.com
Ideal Gas Law — Overview & Calculations Expii Gas Law Equation Sheet the ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. the ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. the ideal gas equation contains five terms, the gas constant r and the variable properties p, v, n, and. Gas Law Equation Sheet.
From docs.google.com
Gas Laws cheat sheet.docx Google Docs Gas Law Equation Sheet the ideal and combined gas laws pv = nrt or p1v1 = p2v2. Use your knowledge of the ideal and combined gas laws to solve the following problems. the ideal gas equation contains five terms, the gas constant r and the variable properties p, v, n, and t. Specifying any four of these terms will permit use of. Gas Law Equation Sheet.
From learningmagicharlow.z13.web.core.windows.net
Formulas For Gas Laws Gas Law Equation Sheet Specifying any four of these terms will permit use of the ideal gas law to calculate the fifth term as demonstrated in the following example exercises. the ideal gas law relates the pressure, temperature, volume, and mass of a gas through the gas constant “r”. the ideal gas equation contains five terms, the gas constant r and the. Gas Law Equation Sheet.