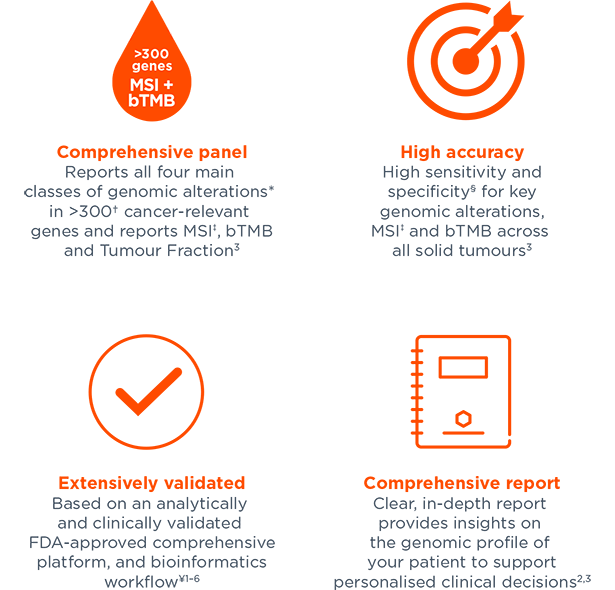
Foundation Medicine Fda . The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. (nasdaq:fmi) today announced that the u.s. 150 second street, cambridge, ma 02141.
from www.foundationmedicine.co.uk
(nasdaq:fmi) today announced that the u.s. The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. 150 second street, cambridge, ma 02141.
FoundationOne Liquid CDx
Foundation Medicine Fda 150 second street, cambridge, ma 02141. (nasdaq:fmi) today announced that the u.s. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. 150 second street, cambridge, ma 02141. The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput.
From nebula.org
Foundation Medicine review 7 facts you should know [DEC 2021] Foundation Medicine Fda The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. (nasdaq:fmi) today announced that the u.s. 150 second street, cambridge, ma 02141. The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. Foundation Medicine Fda.
From www.precisionmedicineonline.com
Foundation Medicine Unveils Strategy to Support Cancer CDx Filings with Foundation Medicine Fda The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. 150 second street, cambridge, ma 02141. (nasdaq:fmi) today announced that the u.s. Foundation Medicine Fda.
From raportuldegarda.ro
FDA a aprobat pembrolizumab împreună cu testul FoundationOne CDx Foundation Medicine Fda 150 second street, cambridge, ma 02141. (nasdaq:fmi) today announced that the u.s. The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. Foundation Medicine Fda.
From www.fusfoundation.org
FDA Pathways to Expedite Patient Access to Innovative Medical Device Foundation Medicine Fda The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. (nasdaq:fmi) today announced that the u.s. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. 150 second street, cambridge, ma 02141. Foundation Medicine Fda.
From www.bjmo.be
FoundationOne CDx receives FDA approval for nonsmall cell lung cancer Foundation Medicine Fda The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. 150 second street, cambridge, ma 02141. The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. (nasdaq:fmi) today announced that the u.s. Foundation Medicine Fda.
From www.bioworld.com
FDA gives thumbs up to Foundationone Liquid CDx 20200827 BioWorld Foundation Medicine Fda (nasdaq:fmi) today announced that the u.s. 150 second street, cambridge, ma 02141. The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. Foundation Medicine Fda.
From raportuldegarda.ro
FDA aprobă testul de biopsie lichidă FoundationOne Liquid CDx ca test Foundation Medicine Fda The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. 150 second street, cambridge, ma 02141. (nasdaq:fmi) today announced that the u.s. Foundation Medicine Fda.
From www.businesswire.com
Foundation Medicine Receives FDA Approval for FoundationOne®CDx as the Foundation Medicine Fda 150 second street, cambridge, ma 02141. (nasdaq:fmi) today announced that the u.s. The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. Foundation Medicine Fda.
From www.foundationmedicine.com
Biopharmaceutical Partnerships Foundation Medicine Foundation Medicine Fda The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. (nasdaq:fmi) today announced that the u.s. 150 second street, cambridge, ma 02141. Foundation Medicine Fda.
From clpmag.com
FDA Approves FoundationOne CDx for Use with Bile Duct Cancer Treatment Foundation Medicine Fda (nasdaq:fmi) today announced that the u.s. The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. 150 second street, cambridge, ma 02141. Foundation Medicine Fda.
From www.researchandmarkets.com
Foundation Medicine Inc Product Pipeline Analysis, 2021 Update Foundation Medicine Fda (nasdaq:fmi) today announced that the u.s. The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. 150 second street, cambridge, ma 02141. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. Foundation Medicine Fda.
From www.foundationmedicine.com
Companion Diagnostics Explained Their Critical Role in Cancer Care and Foundation Medicine Fda The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. 150 second street, cambridge, ma 02141. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. (nasdaq:fmi) today announced that the u.s. Foundation Medicine Fda.
From www.nsmedicaldevices.com
FDA approves FoundationOne CDx as companion diagnostic for Pemazyre Foundation Medicine Fda 150 second street, cambridge, ma 02141. (nasdaq:fmi) today announced that the u.s. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. Foundation Medicine Fda.
From www.healio.com
FDA approves Foundation Medicine pantumor liquid biopsy test Foundation Medicine Fda The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. (nasdaq:fmi) today announced that the u.s. 150 second street, cambridge, ma 02141. Foundation Medicine Fda.
From www.oncozine.com
FoundationOne®Liquid CDx Companion Diagnostic Approved in NSCLC Foundation Medicine Fda The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. (nasdaq:fmi) today announced that the u.s. 150 second street, cambridge, ma 02141. The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. Foundation Medicine Fda.
From www.fda.gov
FoundationOne CDx P170019/S014 FDA Foundation Medicine Fda The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. 150 second street, cambridge, ma 02141. (nasdaq:fmi) today announced that the u.s. Foundation Medicine Fda.
From clpmag.com
FDA Approves New FoundationOne Liquid CDx Companion Diagnostic Foundation Medicine Fda The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. 150 second street, cambridge, ma 02141. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. (nasdaq:fmi) today announced that the u.s. Foundation Medicine Fda.
From www.fda.gov.ph
FDA Advisory No. 20201352 Public Health Warning Against the Foundation Medicine Fda 150 second street, cambridge, ma 02141. The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. (nasdaq:fmi) today announced that the u.s. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. Foundation Medicine Fda.
From www.linkedin.com
Foundation Medicine on LinkedIn The latest indication approval from Foundation Medicine Fda 150 second street, cambridge, ma 02141. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. (nasdaq:fmi) today announced that the u.s. Foundation Medicine Fda.
From www.igenewiki.com
Foundation Medicine,FDA批准重排胆管癌患者伴随诊断方案 医纬(iGeneWiki) Foundation Medicine Fda The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. 150 second street, cambridge, ma 02141. (nasdaq:fmi) today announced that the u.s. Foundation Medicine Fda.
From www.youtube.com
The Foundation Medicine Approach to Personalized Cancer Treatment YouTube Foundation Medicine Fda The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. (nasdaq:fmi) today announced that the u.s. 150 second street, cambridge, ma 02141. Foundation Medicine Fda.
From voice.ons.org
The Names of Targeted Therapies Give Clues to How They Work ONS Voice Foundation Medicine Fda (nasdaq:fmi) today announced that the u.s. The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. 150 second street, cambridge, ma 02141. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. Foundation Medicine Fda.
From www.linkedin.com
Foundation Medicine on LinkedIn FoundationOne CDx* is now FDAapproved Foundation Medicine Fda The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. (nasdaq:fmi) today announced that the u.s. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. 150 second street, cambridge, ma 02141. Foundation Medicine Fda.
From www.yoonsupchoi.com
Foundation Medicine, 점차 확대되는 암 유전체 의학 최윤섭의 Healthcare Innovation Foundation Medicine Fda 150 second street, cambridge, ma 02141. (nasdaq:fmi) today announced that the u.s. The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. Foundation Medicine Fda.
From www.bizjournals.com
Liquid cancer biopsy, bloodbased diagnostic test by Foundation Foundation Medicine Fda 150 second street, cambridge, ma 02141. (nasdaq:fmi) today announced that the u.s. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. Foundation Medicine Fda.
From clpmag.com
FDA Approves FoundationOne CDx as Companion Diagnostic to Detect NTRK Foundation Medicine Fda The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. (nasdaq:fmi) today announced that the u.s. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. 150 second street, cambridge, ma 02141. Foundation Medicine Fda.
From seekingalpha.com
Foundation Medicine To Profit From The Increasing Trend Of Foundation Medicine Fda The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. 150 second street, cambridge, ma 02141. (nasdaq:fmi) today announced that the u.s. The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. Foundation Medicine Fda.
From seekingalpha.com
Foundation Medicine To Profit From The Increasing Trend Of Foundation Medicine Fda The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. (nasdaq:fmi) today announced that the u.s. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. 150 second street, cambridge, ma 02141. Foundation Medicine Fda.
From www.businesswire.com
Incyte and Foundation Medicine Announce Agreement to Develop Companion Foundation Medicine Fda 150 second street, cambridge, ma 02141. The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. (nasdaq:fmi) today announced that the u.s. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. Foundation Medicine Fda.
From www.foundationmedicine.co.uk
FoundationOne Liquid CDx Foundation Medicine Fda 150 second street, cambridge, ma 02141. The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. (nasdaq:fmi) today announced that the u.s. Foundation Medicine Fda.
From www.foundationmedicine.co.nz
FoundationOne Liquid CDx FDAApproved Liquid Biopsy Foundation Medicine Fda 150 second street, cambridge, ma 02141. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. (nasdaq:fmi) today announced that the u.s. Foundation Medicine Fda.
From vetmed.ucsd.edu
Federal Regulatory Agencies Foundation Medicine Fda The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. 150 second street, cambridge, ma 02141. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. (nasdaq:fmi) today announced that the u.s. Foundation Medicine Fda.
From www.businesswire.com
FDA Approves Foundation Medicine’s FoundationOne CDx™, the First and Foundation Medicine Fda 150 second street, cambridge, ma 02141. (nasdaq:fmi) today announced that the u.s. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. Foundation Medicine Fda.
From www.pharmashots.com
Foundation Medicine's FoundationOne CDx Receives FDA's Approval as a Foundation Medicine Fda The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. (nasdaq:fmi) today announced that the u.s. The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. 150 second street, cambridge, ma 02141. Foundation Medicine Fda.
From www.farmfoundation.org
Flyer on New FDA Antibiotics Guidance Available Farm Foundation Foundation Medicine Fda The center for devices and radiological health (cdrh) of the food and drug administration (fda) has completed its review of your premarket. 150 second street, cambridge, ma 02141. The device is a qualitative next generation sequencing based in vitro diagnostic test that uses targeted high throughput. (nasdaq:fmi) today announced that the u.s. Foundation Medicine Fda.