Medical Devices Banned By Fda . (2) medical device classification and regulatory controls,. Food and drug administration (fda) is proposing a ban of electrical stimulation devices (esds) intended. The chronology here highlights milestones in the history of medical device legislation in the united states. Specifically, section 516 of the fd&c act provides that fda may ban a device intended for human use if the agency. The us food and drug administration is again proposing a ban on electrical stimulation devices used to reduce or. In 2011, the institute of medicine (iom) reviewed the fda’s 510(k) clearance process for medical devices, saying it should. This report describes (1) fda’s authority to regulate medical devices; In the first challenge ever brought against fda’s rarely used power to ban a medical device, a court found fda overstepped its.
from journal.com.ph
This report describes (1) fda’s authority to regulate medical devices; In the first challenge ever brought against fda’s rarely used power to ban a medical device, a court found fda overstepped its. In 2011, the institute of medicine (iom) reviewed the fda’s 510(k) clearance process for medical devices, saying it should. Food and drug administration (fda) is proposing a ban of electrical stimulation devices (esds) intended. The chronology here highlights milestones in the history of medical device legislation in the united states. (2) medical device classification and regulatory controls,. Specifically, section 516 of the fd&c act provides that fda may ban a device intended for human use if the agency. The us food and drug administration is again proposing a ban on electrical stimulation devices used to reduce or.
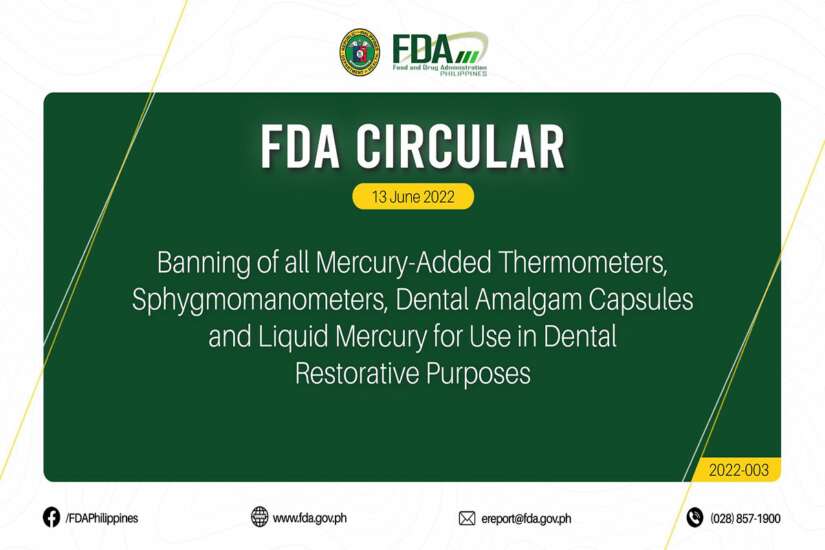
Environmental Group Lauds DOHFDA For Banning Mercury Use In Medical
Medical Devices Banned By Fda Food and drug administration (fda) is proposing a ban of electrical stimulation devices (esds) intended. (2) medical device classification and regulatory controls,. This report describes (1) fda’s authority to regulate medical devices; The chronology here highlights milestones in the history of medical device legislation in the united states. In the first challenge ever brought against fda’s rarely used power to ban a medical device, a court found fda overstepped its. In 2011, the institute of medicine (iom) reviewed the fda’s 510(k) clearance process for medical devices, saying it should. Specifically, section 516 of the fd&c act provides that fda may ban a device intended for human use if the agency. Food and drug administration (fda) is proposing a ban of electrical stimulation devices (esds) intended. The us food and drug administration is again proposing a ban on electrical stimulation devices used to reduce or.
From www.greenlight.guru
FDA Cleared vs Approved vs Granted for Medical Devices Medical Devices Banned By Fda This report describes (1) fda’s authority to regulate medical devices; The us food and drug administration is again proposing a ban on electrical stimulation devices used to reduce or. (2) medical device classification and regulatory controls,. The chronology here highlights milestones in the history of medical device legislation in the united states. Specifically, section 516 of the fd&c act provides. Medical Devices Banned By Fda.
From www.youtube.com
Classification of Medical devices / FDA regulations/ Example of Medical Medical Devices Banned By Fda In 2011, the institute of medicine (iom) reviewed the fda’s 510(k) clearance process for medical devices, saying it should. In the first challenge ever brought against fda’s rarely used power to ban a medical device, a court found fda overstepped its. Specifically, section 516 of the fd&c act provides that fda may ban a device intended for human use if. Medical Devices Banned By Fda.
From ramtechno.com
FDA Abbreviations for Medical Device Designers RAM Technologies Medical Devices Banned By Fda In 2011, the institute of medicine (iom) reviewed the fda’s 510(k) clearance process for medical devices, saying it should. Food and drug administration (fda) is proposing a ban of electrical stimulation devices (esds) intended. In the first challenge ever brought against fda’s rarely used power to ban a medical device, a court found fda overstepped its. The chronology here highlights. Medical Devices Banned By Fda.
From www.fda.gov.ph
FDA Advisory No.20210696 Public Health Warning Against the Purchase Medical Devices Banned By Fda (2) medical device classification and regulatory controls,. In the first challenge ever brought against fda’s rarely used power to ban a medical device, a court found fda overstepped its. The us food and drug administration is again proposing a ban on electrical stimulation devices used to reduce or. This report describes (1) fda’s authority to regulate medical devices; Specifically, section. Medical Devices Banned By Fda.
From www.coolblue.com
What’s the Difference? FDA Form 483 Observations and Warning Letters Medical Devices Banned By Fda Specifically, section 516 of the fd&c act provides that fda may ban a device intended for human use if the agency. This report describes (1) fda’s authority to regulate medical devices; The us food and drug administration is again proposing a ban on electrical stimulation devices used to reduce or. Food and drug administration (fda) is proposing a ban of. Medical Devices Banned By Fda.
From blogs.blackberry.com
New FDA Medical Device Cybersecurity Requirements and How to Simplify Medical Devices Banned By Fda In 2011, the institute of medicine (iom) reviewed the fda’s 510(k) clearance process for medical devices, saying it should. Food and drug administration (fda) is proposing a ban of electrical stimulation devices (esds) intended. (2) medical device classification and regulatory controls,. In the first challenge ever brought against fda’s rarely used power to ban a medical device, a court found. Medical Devices Banned By Fda.
From www.youtube.com
Medical Device Regulations / FDA Approval YouTube Medical Devices Banned By Fda In 2011, the institute of medicine (iom) reviewed the fda’s 510(k) clearance process for medical devices, saying it should. The chronology here highlights milestones in the history of medical device legislation in the united states. (2) medical device classification and regulatory controls,. This report describes (1) fda’s authority to regulate medical devices; Specifically, section 516 of the fd&c act provides. Medical Devices Banned By Fda.
From www.regdesk.co
FDA Guidance on Medical Device Reporting Written Procedures, Record Medical Devices Banned By Fda The chronology here highlights milestones in the history of medical device legislation in the united states. In 2011, the institute of medicine (iom) reviewed the fda’s 510(k) clearance process for medical devices, saying it should. The us food and drug administration is again proposing a ban on electrical stimulation devices used to reduce or. (2) medical device classification and regulatory. Medical Devices Banned By Fda.
From bantoxics.org
BAN Toxics applauds FDA for the issuance of Health Warning Against Medical Devices Banned By Fda (2) medical device classification and regulatory controls,. Food and drug administration (fda) is proposing a ban of electrical stimulation devices (esds) intended. This report describes (1) fda’s authority to regulate medical devices; The us food and drug administration is again proposing a ban on electrical stimulation devices used to reduce or. The chronology here highlights milestones in the history of. Medical Devices Banned By Fda.
From www.nsmedicaldevices.com
Seven major medical devices approved by the US FDA in 2020 Medical Devices Banned By Fda Food and drug administration (fda) is proposing a ban of electrical stimulation devices (esds) intended. In the first challenge ever brought against fda’s rarely used power to ban a medical device, a court found fda overstepped its. The chronology here highlights milestones in the history of medical device legislation in the united states. The us food and drug administration is. Medical Devices Banned By Fda.
From medworksadvantage.com
FDA's Role in Regulating Medical Devices Ensuring Safety and Efficacy Medical Devices Banned By Fda Specifically, section 516 of the fd&c act provides that fda may ban a device intended for human use if the agency. This report describes (1) fda’s authority to regulate medical devices; The us food and drug administration is again proposing a ban on electrical stimulation devices used to reduce or. The chronology here highlights milestones in the history of medical. Medical Devices Banned By Fda.
From www.regdesk.co
FDA on Medical Device Reporting Specific Aspects RegDesk Medical Devices Banned By Fda Specifically, section 516 of the fd&c act provides that fda may ban a device intended for human use if the agency. This report describes (1) fda’s authority to regulate medical devices; (2) medical device classification and regulatory controls,. The us food and drug administration is again proposing a ban on electrical stimulation devices used to reduce or. The chronology here. Medical Devices Banned By Fda.
From www.drugwatch.com
Overview of the Safe Medical Devices Act of 1990 Medical Devices Banned By Fda Food and drug administration (fda) is proposing a ban of electrical stimulation devices (esds) intended. Specifically, section 516 of the fd&c act provides that fda may ban a device intended for human use if the agency. The chronology here highlights milestones in the history of medical device legislation in the united states. In the first challenge ever brought against fda’s. Medical Devices Banned By Fda.
From journal.com.ph
BAN Toxics Applauds FDA For The Issuance Of Health Warning Against Medical Devices Banned By Fda Food and drug administration (fda) is proposing a ban of electrical stimulation devices (esds) intended. In the first challenge ever brought against fda’s rarely used power to ban a medical device, a court found fda overstepped its. (2) medical device classification and regulatory controls,. Specifically, section 516 of the fd&c act provides that fda may ban a device intended for. Medical Devices Banned By Fda.
From www.slideshare.net
Export Certificates for Medical Devices Medical Devices Banned By Fda This report describes (1) fda’s authority to regulate medical devices; In 2011, the institute of medicine (iom) reviewed the fda’s 510(k) clearance process for medical devices, saying it should. The chronology here highlights milestones in the history of medical device legislation in the united states. Specifically, section 516 of the fd&c act provides that fda may ban a device intended. Medical Devices Banned By Fda.
From www.medtechimpact.com
FDA Focuses on Medical Device Safety • Medtech Impact On Wellness Medical Devices Banned By Fda Specifically, section 516 of the fd&c act provides that fda may ban a device intended for human use if the agency. The us food and drug administration is again proposing a ban on electrical stimulation devices used to reduce or. (2) medical device classification and regulatory controls,. The chronology here highlights milestones in the history of medical device legislation in. Medical Devices Banned By Fda.
From medicalclaimlegal.com
Powdered Medical Gloves Banned By the FDA Medical Devices Banned By Fda The chronology here highlights milestones in the history of medical device legislation in the united states. The us food and drug administration is again proposing a ban on electrical stimulation devices used to reduce or. (2) medical device classification and regulatory controls,. Food and drug administration (fda) is proposing a ban of electrical stimulation devices (esds) intended. Specifically, section 516. Medical Devices Banned By Fda.
From journal.com.ph
Environmental Group Lauds DOHFDA For Banning Mercury Use In Medical Medical Devices Banned By Fda In the first challenge ever brought against fda’s rarely used power to ban a medical device, a court found fda overstepped its. Specifically, section 516 of the fd&c act provides that fda may ban a device intended for human use if the agency. The chronology here highlights milestones in the history of medical device legislation in the united states. The. Medical Devices Banned By Fda.
From vem-medical.com
Medical Device Manufacturing Medical Devices Banned By Fda Specifically, section 516 of the fd&c act provides that fda may ban a device intended for human use if the agency. This report describes (1) fda’s authority to regulate medical devices; The us food and drug administration is again proposing a ban on electrical stimulation devices used to reduce or. The chronology here highlights milestones in the history of medical. Medical Devices Banned By Fda.
From edition.cnn.com
Some medical devices are susceptible to hackers, FDA warns CNN Medical Devices Banned By Fda In the first challenge ever brought against fda’s rarely used power to ban a medical device, a court found fda overstepped its. Food and drug administration (fda) is proposing a ban of electrical stimulation devices (esds) intended. In 2011, the institute of medicine (iom) reviewed the fda’s 510(k) clearance process for medical devices, saying it should. Specifically, section 516 of. Medical Devices Banned By Fda.
From www.careersinfosecurity.com
FDA Reacts to Critique of Medical Device Security Strategy Medical Devices Banned By Fda The chronology here highlights milestones in the history of medical device legislation in the united states. Food and drug administration (fda) is proposing a ban of electrical stimulation devices (esds) intended. This report describes (1) fda’s authority to regulate medical devices; In 2011, the institute of medicine (iom) reviewed the fda’s 510(k) clearance process for medical devices, saying it should.. Medical Devices Banned By Fda.
From www.bankinfosecurity.com
FDA Will Begin Rejecting Medical Devices Over Cyber Soon Medical Devices Banned By Fda Specifically, section 516 of the fd&c act provides that fda may ban a device intended for human use if the agency. The us food and drug administration is again proposing a ban on electrical stimulation devices used to reduce or. Food and drug administration (fda) is proposing a ban of electrical stimulation devices (esds) intended. (2) medical device classification and. Medical Devices Banned By Fda.
From ramtechno.com
FDA vs. EU Medical Device Regulation RAM Technologies Medical Devices Banned By Fda The chronology here highlights milestones in the history of medical device legislation in the united states. This report describes (1) fda’s authority to regulate medical devices; In the first challenge ever brought against fda’s rarely used power to ban a medical device, a court found fda overstepped its. The us food and drug administration is again proposing a ban on. Medical Devices Banned By Fda.
From premier-research.com
Medical Device Trials What You Need to Know About U.S. Regulations Medical Devices Banned By Fda The chronology here highlights milestones in the history of medical device legislation in the united states. This report describes (1) fda’s authority to regulate medical devices; In 2011, the institute of medicine (iom) reviewed the fda’s 510(k) clearance process for medical devices, saying it should. (2) medical device classification and regulatory controls,. Food and drug administration (fda) is proposing a. Medical Devices Banned By Fda.
From www.complianceteamllc.com
Pharmaceutical Compliance Services Compliance Team Regulatory Consultants Medical Devices Banned By Fda In the first challenge ever brought against fda’s rarely used power to ban a medical device, a court found fda overstepped its. The chronology here highlights milestones in the history of medical device legislation in the united states. Food and drug administration (fda) is proposing a ban of electrical stimulation devices (esds) intended. The us food and drug administration is. Medical Devices Banned By Fda.
From www.regdesk.co
FDA Guidance on Development of Medical Device Labeling RegDesk Medical Devices Banned By Fda In 2011, the institute of medicine (iom) reviewed the fda’s 510(k) clearance process for medical devices, saying it should. The chronology here highlights milestones in the history of medical device legislation in the united states. The us food and drug administration is again proposing a ban on electrical stimulation devices used to reduce or. In the first challenge ever brought. Medical Devices Banned By Fda.
From www.youtube.com
Medical Devices classification as per FDA Medical Device Regulations Medical Devices Banned By Fda Food and drug administration (fda) is proposing a ban of electrical stimulation devices (esds) intended. The us food and drug administration is again proposing a ban on electrical stimulation devices used to reduce or. (2) medical device classification and regulatory controls,. Specifically, section 516 of the fd&c act provides that fda may ban a device intended for human use if. Medical Devices Banned By Fda.
From iasnext.com
National Medical Devices Policy 2023 Current Affairs Medical Devices Banned By Fda In the first challenge ever brought against fda’s rarely used power to ban a medical device, a court found fda overstepped its. The chronology here highlights milestones in the history of medical device legislation in the united states. The us food and drug administration is again proposing a ban on electrical stimulation devices used to reduce or. In 2011, the. Medical Devices Banned By Fda.
From www.regdesk.co
FDA on Reusable Medical Devices and Reprocessing RegDesk Medical Devices Banned By Fda In 2011, the institute of medicine (iom) reviewed the fda’s 510(k) clearance process for medical devices, saying it should. The chronology here highlights milestones in the history of medical device legislation in the united states. This report describes (1) fda’s authority to regulate medical devices; Food and drug administration (fda) is proposing a ban of electrical stimulation devices (esds) intended.. Medical Devices Banned By Fda.
From bantoxics.org
Environmental group lauds DOHFDA for banning mercury use in medical Medical Devices Banned By Fda The chronology here highlights milestones in the history of medical device legislation in the united states. This report describes (1) fda’s authority to regulate medical devices; Specifically, section 516 of the fd&c act provides that fda may ban a device intended for human use if the agency. In 2011, the institute of medicine (iom) reviewed the fda’s 510(k) clearance process. Medical Devices Banned By Fda.
From www.slideshare.net
Medical Device FDA Regulations and Classifications infographic Medical Devices Banned By Fda This report describes (1) fda’s authority to regulate medical devices; In the first challenge ever brought against fda’s rarely used power to ban a medical device, a court found fda overstepped its. (2) medical device classification and regulatory controls,. In 2011, the institute of medicine (iom) reviewed the fda’s 510(k) clearance process for medical devices, saying it should. The us. Medical Devices Banned By Fda.
From www.presentationeze.com
Medical Device Regulations. Design Requirements PresentationEZE Medical Devices Banned By Fda In 2011, the institute of medicine (iom) reviewed the fda’s 510(k) clearance process for medical devices, saying it should. The us food and drug administration is again proposing a ban on electrical stimulation devices used to reduce or. Food and drug administration (fda) is proposing a ban of electrical stimulation devices (esds) intended. This report describes (1) fda’s authority to. Medical Devices Banned By Fda.
From sterlingmedicaldevices.com
FDA Medical Device Regulation Guidance for 2022 Medical Devices Banned By Fda Food and drug administration (fda) is proposing a ban of electrical stimulation devices (esds) intended. This report describes (1) fda’s authority to regulate medical devices; (2) medical device classification and regulatory controls,. The us food and drug administration is again proposing a ban on electrical stimulation devices used to reduce or. In the first challenge ever brought against fda’s rarely. Medical Devices Banned By Fda.
From scrolller.com
A “medical device” that tortures disabled people with electric shocks Medical Devices Banned By Fda Specifically, section 516 of the fd&c act provides that fda may ban a device intended for human use if the agency. The us food and drug administration is again proposing a ban on electrical stimulation devices used to reduce or. This report describes (1) fda’s authority to regulate medical devices; The chronology here highlights milestones in the history of medical. Medical Devices Banned By Fda.
From safepatientadvocate.com
For the second time in its history, FDA bans a medical device (powdered Medical Devices Banned By Fda The chronology here highlights milestones in the history of medical device legislation in the united states. In 2011, the institute of medicine (iom) reviewed the fda’s 510(k) clearance process for medical devices, saying it should. (2) medical device classification and regulatory controls,. The us food and drug administration is again proposing a ban on electrical stimulation devices used to reduce. Medical Devices Banned By Fda.