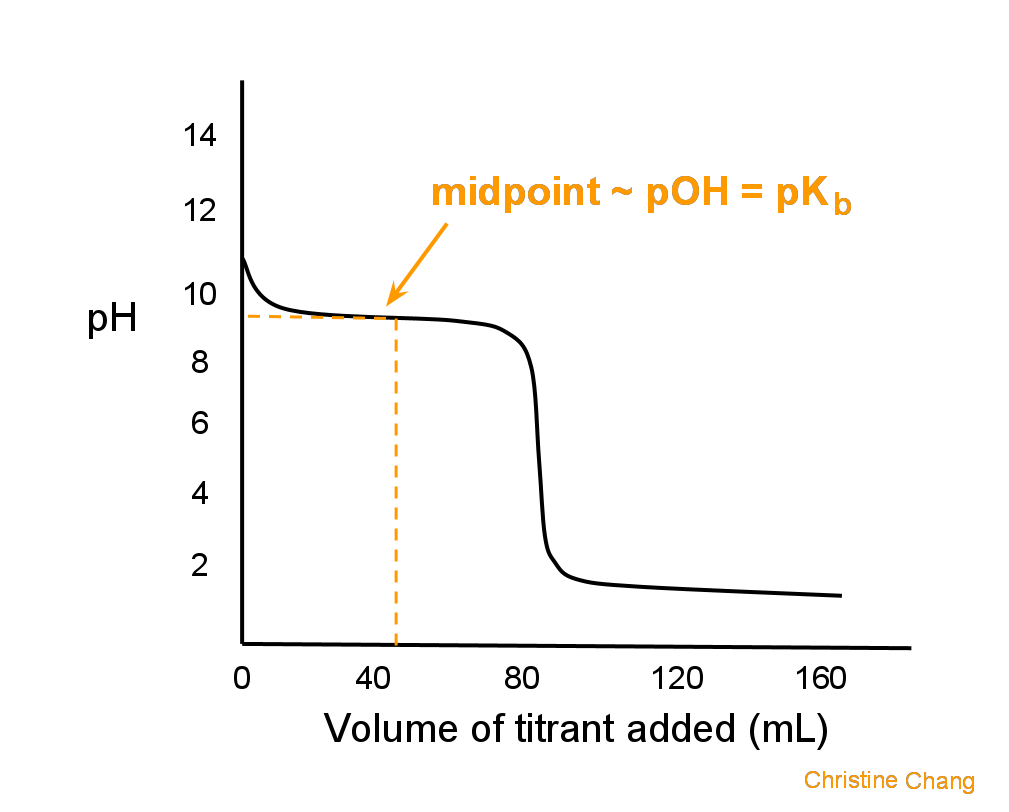
Titration Curve Function . a titration curve is a graphical representation of the ph of a solution during a titration. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. figure \(\pageindex{4}\) illustrates the shape of titration curves as a function of the \(pk_a\) or the \(pk_b\). a titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different. titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. the titration curve is a graphical representation of the ph or other property changes during a titration experiment. On the left is a titration in which the base is added to the acid, and so the ph progresses from low to high. It provides valuable information about the reaction under study and helps understand the results obtained. The end point of a titration is the point at.
from chem.libretexts.org
the titration curve is a graphical representation of the ph or other property changes during a titration experiment. figure \(\pageindex{4}\) illustrates the shape of titration curves as a function of the \(pk_a\) or the \(pk_b\). a titration curve is a graphical representation of the ph of a solution during a titration. a titration curve is a graphical representation of the ph of a solution during a titration. titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. It provides valuable information about the reaction under study and helps understand the results obtained. The figure below shows two different. The end point of a titration is the point at. On the left is a titration in which the base is added to the acid, and so the ph progresses from low to high. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it.
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts
Titration Curve Function On the left is a titration in which the base is added to the acid, and so the ph progresses from low to high. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The figure below shows two different. a titration curve is a graphical representation of the ph of a solution during a titration. It provides valuable information about the reaction under study and helps understand the results obtained. titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. a titration curve is a graphical representation of the ph of a solution during a titration. On the left is a titration in which the base is added to the acid, and so the ph progresses from low to high. the titration curve is a graphical representation of the ph or other property changes during a titration experiment. The end point of a titration is the point at. figure \(\pageindex{4}\) illustrates the shape of titration curves as a function of the \(pk_a\) or the \(pk_b\).
From www.reddit.com
How to find concentration from a titration curve? r/chemistryhelp Titration Curve Function a titration curve is a graphical representation of the ph of a solution during a titration. The end point of a titration is the point at. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. the titration curve is a graphical representation of. Titration Curve Function.
From pressbooks.bccampus.ca
14.7 AcidBase Titrations Chemistry 2e for Chem 120 (Chemistry for Titration Curve Function The figure below shows two different. figure \(\pageindex{4}\) illustrates the shape of titration curves as a function of the \(pk_a\) or the \(pk_b\). The end point of a titration is the point at. the titration curve is a graphical representation of the ph or other property changes during a titration experiment. On the left is a titration in. Titration Curve Function.
From exoyzonai.blob.core.windows.net
Titration Curve Labeled Buffer Region at Craig Johnson blog Titration Curve Function titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. It provides valuable information about the reaction under study and helps understand the results obtained. The figure below shows two different. figure \(\pageindex{4}\) illustrates the shape of titration curves as a function of the \(pk_a\) or the \(pk_b\). . Titration Curve Function.
From saylordotorg.github.io
AcidBase Titrations Titration Curve Function the titration curve is a graphical representation of the ph or other property changes during a titration experiment. It provides valuable information about the reaction under study and helps understand the results obtained. a titration curve is a graphical representation of the ph of a solution during a titration. titrations are often recorded on graphs called titration. Titration Curve Function.
From www.researchgate.net
Typical amperometric titration curves where (A) only analyte is Titration Curve Function a titration curve is a graphical representation of the ph of a solution during a titration. titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. a titration curve is a graphical representation of the ph of a solution during a titration. figure \(\pageindex{4}\) illustrates the shape. Titration Curve Function.
From psu.pb.unizin.org
AcidBase Titrations (14.7) Chemistry 110 Titration Curve Function On the left is a titration in which the base is added to the acid, and so the ph progresses from low to high. a titration curve is a graphical representation of the ph of a solution during a titration. It provides valuable information about the reaction under study and helps understand the results obtained. the titration curve. Titration Curve Function.
From www.researchgate.net
Conductivity titration curve of the chitosan sample (A), analytical Titration Curve Function titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. The end point of a titration is the point at. figure \(\pageindex{4}\) illustrates the shape of titration curves as a function of the \(pk_a\) or the \(pk_b\). the titration curve is a graphical representation of the ph or. Titration Curve Function.
From www.pearson.com
The graphs labeled (a) and (b) show the titration curves for two Titration Curve Function On the left is a titration in which the base is added to the acid, and so the ph progresses from low to high. It provides valuable information about the reaction under study and helps understand the results obtained. the titration curve is a graphical representation of the ph or other property changes during a titration experiment. figure. Titration Curve Function.
From www.albert.io
[HF] and [F^] Comparison from a Titration Curve AP® Chemistry Titration Curve Function It provides valuable information about the reaction under study and helps understand the results obtained. The end point of a titration is the point at. figure \(\pageindex{4}\) illustrates the shape of titration curves as a function of the \(pk_a\) or the \(pk_b\). On the left is a titration in which the base is added to the acid, and so. Titration Curve Function.
From www.youtube.com
AP Chem unit 8.5 Titration Curves YouTube Titration Curve Function The end point of a titration is the point at. titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. figure \(\pageindex{4}\) illustrates the shape of titration curves as a function of the \(pk_a\) or the \(pk_b\). a titration curve is a graphical representation of the ph of. Titration Curve Function.
From www.researchgate.net
Potentiometric titration curves of the semiquinone g 2.00 signal Titration Curve Function On the left is a titration in which the base is added to the acid, and so the ph progresses from low to high. the titration curve is a graphical representation of the ph or other property changes during a titration experiment. a titration curve is a graphical representation of the ph of a solution during a titration.. Titration Curve Function.
From www.chemistrystudent.com
Titration Curves (ALevel) ChemistryStudent Titration Curve Function On the left is a titration in which the base is added to the acid, and so the ph progresses from low to high. The figure below shows two different. The end point of a titration is the point at. figure \(\pageindex{4}\) illustrates the shape of titration curves as a function of the \(pk_a\) or the \(pk_b\). titration. Titration Curve Function.
From www.expii.com
What Is a Titration Curve? — Overview & Parts Expii Titration Curve Function On the left is a titration in which the base is added to the acid, and so the ph progresses from low to high. It provides valuable information about the reaction under study and helps understand the results obtained. figure \(\pageindex{4}\) illustrates the shape of titration curves as a function of the \(pk_a\) or the \(pk_b\). a titration. Titration Curve Function.
From www.researchgate.net
The theoretical titration curves and Gran functions for titration of Titration Curve Function titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. The end point of a titration is the point at. The figure below shows two different. It provides valuable information about the reaction under study and helps understand the results obtained. titration curves show how the ph of an. Titration Curve Function.
From chemwiki.ucdavis.edu
9B AcidBase Titrations Chemwiki Titration Curve Function titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. the titration curve is a graphical representation of the ph or other property changes during a titration experiment. a titration curve is a graphical representation of the ph of a solution during a titration.. Titration Curve Function.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Titration Curve Function titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. the titration curve is a graphical representation of the ph or other property changes during a titration experiment. On the left is a titration in which the base is added to the acid, and so the ph progresses from. Titration Curve Function.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Curve Function a titration curve is a graphical representation of the ph of a solution during a titration. It provides valuable information about the reaction under study and helps understand the results obtained. The figure below shows two different. The end point of a titration is the point at. On the left is a titration in which the base is added. Titration Curve Function.
From chem.libretexts.org
15.6 AcidBase Titration Curves Chemistry LibreTexts Titration Curve Function It provides valuable information about the reaction under study and helps understand the results obtained. a titration curve is a graphical representation of the ph of a solution during a titration. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. titrations are often. Titration Curve Function.
From chemistryguru.com.sg
Titration Curve of Amino Acid Titration Curve Function titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. It provides valuable information about the reaction under study and helps understand the results obtained. . Titration Curve Function.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Titration Curve Function the titration curve is a graphical representation of the ph or other property changes during a titration experiment. a titration curve is a graphical representation of the ph of a solution during a titration. figure \(\pageindex{4}\) illustrates the shape of titration curves as a function of the \(pk_a\) or the \(pk_b\). titration curves show how the. Titration Curve Function.
From chem.libretexts.org
9.4 Redox Titrations Chemistry LibreTexts Titration Curve Function the titration curve is a graphical representation of the ph or other property changes during a titration experiment. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. titrations are often recorded on graphs called titration curves, which generally contain the volume of the. Titration Curve Function.
From solvedlib.com
The graph below shows the titration curves for two mo… SolvedLib Titration Curve Function On the left is a titration in which the base is added to the acid, and so the ph progresses from low to high. a titration curve is a graphical representation of the ph of a solution during a titration. titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as. Titration Curve Function.
From www.grace.umd.edu
Simulation of Monoprotic Titration Curve Titration Curve Function a titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different. titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. It provides valuable information about the reaction under study and helps understand the results obtained. On the. Titration Curve Function.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Titration Curve Function titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. a titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different. figure \(\pageindex{4}\) illustrates the shape of titration curves as a function of the \(pk_a\) or the. Titration Curve Function.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Titration Curve Function a titration curve is a graphical representation of the ph of a solution during a titration. a titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different. It provides valuable information about the reaction under study and helps understand the results obtained. The end point of a. Titration Curve Function.
From www.ck12.org
Titration Curve Overview ( Video ) Chemistry CK12 Foundation Titration Curve Function a titration curve is a graphical representation of the ph of a solution during a titration. a titration curve is a graphical representation of the ph of a solution during a titration. On the left is a titration in which the base is added to the acid, and so the ph progresses from low to high. The figure. Titration Curve Function.
From exogrnoza.blob.core.windows.net
Titration Curve Acetic Acid And Naoh at Isabel Keith blog Titration Curve Function figure \(\pageindex{4}\) illustrates the shape of titration curves as a function of the \(pk_a\) or the \(pk_b\). The figure below shows two different. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. The end point of a titration is the point at. It provides. Titration Curve Function.
From chemwiki.ucdavis.edu
9B AcidBase Titrations Chemwiki Titration Curve Function figure \(\pageindex{4}\) illustrates the shape of titration curves as a function of the \(pk_a\) or the \(pk_b\). The end point of a titration is the point at. The figure below shows two different. a titration curve is a graphical representation of the ph of a solution during a titration. titration curves show how the ph of an. Titration Curve Function.
From www.researchgate.net
Theoretical titration curves. The calculated curves are shown in red Titration Curve Function a titration curve is a graphical representation of the ph of a solution during a titration. On the left is a titration in which the base is added to the acid, and so the ph progresses from low to high. It provides valuable information about the reaction under study and helps understand the results obtained. figure \(\pageindex{4}\) illustrates. Titration Curve Function.
From www.vrogue.co
The Graphs Labeled A And B Show The Titration Curves vrogue.co Titration Curve Function The end point of a titration is the point at. On the left is a titration in which the base is added to the acid, and so the ph progresses from low to high. The figure below shows two different. figure \(\pageindex{4}\) illustrates the shape of titration curves as a function of the \(pk_a\) or the \(pk_b\). titration. Titration Curve Function.
From saylordotorg.github.io
AcidBase Titrations Titration Curve Function a titration curve is a graphical representation of the ph of a solution during a titration. The figure below shows two different. titration curves show how the ph of an acidic or basic solution changes as a basic or acidic solution is added to it. On the left is a titration in which the base is added to. Titration Curve Function.
From chem.libretexts.org
Redox Titration Chemistry LibreTexts Titration Curve Function a titration curve is a graphical representation of the ph of a solution during a titration. the titration curve is a graphical representation of the ph or other property changes during a titration experiment. titrations are often recorded on graphs called titration curves, which generally contain the volume of the titrant as the. titration curves show. Titration Curve Function.
From mungfali.com
Titration Curve Labeled Titration Curve Function a titration curve is a graphical representation of the ph of a solution during a titration. the titration curve is a graphical representation of the ph or other property changes during a titration experiment. The figure below shows two different. a titration curve is a graphical representation of the ph of a solution during a titration. . Titration Curve Function.
From generalchemistrylab.blogspot.com
Chemistry Laboratory Titration curve & HendersonHasselbalch equation Titration Curve Function The figure below shows two different. figure \(\pageindex{4}\) illustrates the shape of titration curves as a function of the \(pk_a\) or the \(pk_b\). On the left is a titration in which the base is added to the acid, and so the ph progresses from low to high. titrations are often recorded on graphs called titration curves, which generally. Titration Curve Function.
From byjus.com
Acid Base Titration Titration Curves, Equivalence Point & Indicators Titration Curve Function The figure below shows two different. a titration curve is a graphical representation of the ph of a solution during a titration. The end point of a titration is the point at. a titration curve is a graphical representation of the ph of a solution during a titration. On the left is a titration in which the base. Titration Curve Function.