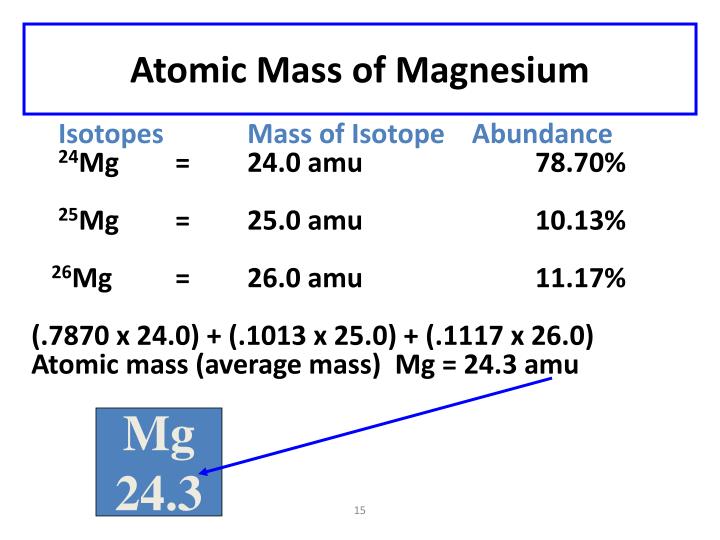
Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes . 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: The symbol for an atom. Magnesium (mg) has three significant natural isotopes: 24 mg (23.985) with 78.99% abundance, 25 mg (24.986) with 10.00% abundance, and a. There are 19 radioisotopes that have been. The weighted average of the individual isotopes is the atomic mass quoted on the periodic table. Because most elements exist as mixtures of several stable isotopes, the atomic mass of an element is defined as the weighted average of the masses of the isotopes. The magnesium isotope has a mass of 24 and a charge of +2, so it is written as 24 mg 2 +. Magnesium has three naturally occurring isotopes: Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the. For stable elements, there is usually a variety of stable isotopes. 78.70% of all magnesium atoms have an atomic weight of 23.985 amu, 10.13% have an.
from www.slideserve.com
46 rows magnesium (12 mg) naturally occurs in three stable isotopes: 78.70% of all magnesium atoms have an atomic weight of 23.985 amu, 10.13% have an. Magnesium has three naturally occurring isotopes: Because most elements exist as mixtures of several stable isotopes, the atomic mass of an element is defined as the weighted average of the masses of the isotopes. There are 19 radioisotopes that have been. For stable elements, there is usually a variety of stable isotopes. 24 mg (23.985) with 78.99% abundance, 25 mg (24.986) with 10.00% abundance, and a. Magnesium (mg) has three significant natural isotopes: Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the. The weighted average of the individual isotopes is the atomic mass quoted on the periodic table.
PPT Atoms, Ions and Isotopes PowerPoint Presentation ID5401475
Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes Because most elements exist as mixtures of several stable isotopes, the atomic mass of an element is defined as the weighted average of the masses of the isotopes. The weighted average of the individual isotopes is the atomic mass quoted on the periodic table. There are 19 radioisotopes that have been. Because most elements exist as mixtures of several stable isotopes, the atomic mass of an element is defined as the weighted average of the masses of the isotopes. Magnesium (mg) has three significant natural isotopes: 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: 78.70% of all magnesium atoms have an atomic weight of 23.985 amu, 10.13% have an. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the. 24 mg (23.985) with 78.99% abundance, 25 mg (24.986) with 10.00% abundance, and a. For stable elements, there is usually a variety of stable isotopes. The symbol for an atom. Magnesium has three naturally occurring isotopes: The magnesium isotope has a mass of 24 and a charge of +2, so it is written as 24 mg 2 +.
From www.animalia-life.club
Magnesium Electron Configuration Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: The symbol for an atom. 78.70% of all magnesium atoms have an atomic weight of 23.985 amu, 10.13% have an. Magnesium (mg) has three significant natural isotopes: Because most elements exist as mixtures of several stable isotopes, the atomic mass of an element is defined as the weighted average. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From brainly.com
2. Using the following data, calculate the average atomic mass of Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes 24 mg (23.985) with 78.99% abundance, 25 mg (24.986) with 10.00% abundance, and a. Magnesium (mg) has three significant natural isotopes: 78.70% of all magnesium atoms have an atomic weight of 23.985 amu, 10.13% have an. 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: Magnesium has three naturally occurring isotopes: Because most elements exist as mixtures of. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From www.vrogue.co
Ppt Isotopes And Atomic Mass What Does The Mass On Th vrogue.co Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the. Magnesium has three naturally occurring isotopes: The symbol for an atom. 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: Because most elements exist as mixtures of several stable isotopes, the atomic mass of an element is defined as. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From kunduz.com
[ANSWERED] Weight of magnesium atomic mass 24 u deposited by a quantity Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes The weighted average of the individual isotopes is the atomic mass quoted on the periodic table. 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: The symbol for an atom. Because most elements exist as mixtures of several stable isotopes, the atomic mass of an element is defined as the weighted average of the masses of the isotopes.. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From ecurrencythailand.com
Which Elements Do Not Have Naturally Occurring Isotopes? Trust The Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes 78.70% of all magnesium atoms have an atomic weight of 23.985 amu, 10.13% have an. There are 19 radioisotopes that have been. Because most elements exist as mixtures of several stable isotopes, the atomic mass of an element is defined as the weighted average of the masses of the isotopes. 24 mg (23.985) with 78.99% abundance, 25 mg (24.986) with. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From www.sliderbase.com
Atomic Mass Magnesium has three isotopes. 78.99 magnesium 24 with a Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes 24 mg (23.985) with 78.99% abundance, 25 mg (24.986) with 10.00% abundance, and a. The magnesium isotope has a mass of 24 and a charge of +2, so it is written as 24 mg 2 +. The symbol for an atom. Magnesium (mg) has three significant natural isotopes: For stable elements, there is usually a variety of stable isotopes. The. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From newtondesk.com
Magnesium Mg (Element 12) of Periodic Table Elements FlashCards Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the. For stable elements, there is usually a variety of stable isotopes. Because most elements exist as mixtures of several stable isotopes, the atomic mass of an element is defined as the weighted average of the masses of the isotopes. There are. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From www.slideserve.com
PPT Isotopes of Magnesium PowerPoint Presentation ID4271266 Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes The symbol for an atom. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the. For stable elements, there is usually a variety of stable isotopes. The weighted average of the individual isotopes is the atomic mass quoted on the periodic table. 24 mg (23.985) with 78.99% abundance, 25 mg (24.986). Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From hunterturninaing.blogspot.com
how to find the average atomic mass Hunter Turninaing Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes Magnesium (mg) has three significant natural isotopes: There are 19 radioisotopes that have been. For stable elements, there is usually a variety of stable isotopes. 78.70% of all magnesium atoms have an atomic weight of 23.985 amu, 10.13% have an. The weighted average of the individual isotopes is the atomic mass quoted on the periodic table. The magnesium isotope has. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From ar.inspiredpencil.com
Magnesium Atom 3d Model Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the. Magnesium (mg) has three significant natural isotopes: 24 mg (23.985) with 78.99% abundance, 25 mg (24.986) with 10.00% abundance, and a. 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: 78.70% of all magnesium atoms have an atomic weight. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes For stable elements, there is usually a variety of stable isotopes. The magnesium isotope has a mass of 24 and a charge of +2, so it is written as 24 mg 2 +. 24 mg (23.985) with 78.99% abundance, 25 mg (24.986) with 10.00% abundance, and a. 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: There are. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From material-properties.org
Magnesium Periodic Table and Atomic Properties Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes The weighted average of the individual isotopes is the atomic mass quoted on the periodic table. The magnesium isotope has a mass of 24 and a charge of +2, so it is written as 24 mg 2 +. The symbol for an atom. There are 19 radioisotopes that have been. Isotopes are nuclides that have the same atomic number and. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From lessonlibrarypaliform.z14.web.core.windows.net
How To Average Atomic Mass Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes 24 mg (23.985) with 78.99% abundance, 25 mg (24.986) with 10.00% abundance, and a. 78.70% of all magnesium atoms have an atomic weight of 23.985 amu, 10.13% have an. The weighted average of the individual isotopes is the atomic mass quoted on the periodic table. Magnesium has three naturally occurring isotopes: The symbol for an atom. There are 19 radioisotopes. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From general.chemistrysteps.com
What Are Isotopes? Chemistry Steps Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes 24 mg (23.985) with 78.99% abundance, 25 mg (24.986) with 10.00% abundance, and a. Because most elements exist as mixtures of several stable isotopes, the atomic mass of an element is defined as the weighted average of the masses of the isotopes. The symbol for an atom. The weighted average of the individual isotopes is the atomic mass quoted on. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From www.slideserve.com
PPT Atoms, Ions and Isotopes PowerPoint Presentation ID5401475 Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes Magnesium has three naturally occurring isotopes: Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the. 78.70% of all magnesium atoms have an atomic weight of 23.985 amu, 10.13% have an. Because most elements exist as mixtures of several stable isotopes, the atomic mass of an element is defined as the. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From www.slideserve.com
PPT The Atom Atomic Number Mass Number Isotopes PowerPoint Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes For stable elements, there is usually a variety of stable isotopes. There are 19 radioisotopes that have been. 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: Because most elements exist as mixtures of several stable isotopes, the atomic mass of an element is defined as the weighted average of the masses of the isotopes. The symbol for. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From slideplayer.com
Magnesium Magnesium Atomic No. 12 Atomic mass ppt download Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes Magnesium has three naturally occurring isotopes: For stable elements, there is usually a variety of stable isotopes. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the. Because most elements exist as mixtures of several stable isotopes, the atomic mass of an element is defined as the weighted average of the. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From ar.inspiredpencil.com
Magnesium Atom Diagram Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes 24 mg (23.985) with 78.99% abundance, 25 mg (24.986) with 10.00% abundance, and a. The magnesium isotope has a mass of 24 and a charge of +2, so it is written as 24 mg 2 +. Because most elements exist as mixtures of several stable isotopes, the atomic mass of an element is defined as the weighted average of the. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From symbiosis.co.nz
Magnesium Symbiosis Agriculture Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes The weighted average of the individual isotopes is the atomic mass quoted on the periodic table. Because most elements exist as mixtures of several stable isotopes, the atomic mass of an element is defined as the weighted average of the masses of the isotopes. 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: 24 mg (23.985) with 78.99%. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From www.wikihow.com
3 Clear and Easy Ways to Calculate Atomic Mass wikiHow Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes The weighted average of the individual isotopes is the atomic mass quoted on the periodic table. The symbol for an atom. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the. There are 19 radioisotopes that have been. The magnesium isotope has a mass of 24 and a charge of +2,. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From www.meritnation.com
I want a labelled diagram of the atom of magnesium not the atomic no or Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes 24 mg (23.985) with 78.99% abundance, 25 mg (24.986) with 10.00% abundance, and a. 78.70% of all magnesium atoms have an atomic weight of 23.985 amu, 10.13% have an. For stable elements, there is usually a variety of stable isotopes. There are 19 radioisotopes that have been. Magnesium (mg) has three significant natural isotopes: The magnesium isotope has a mass. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From periodictable.me
How to Calculate Atomic Mass of Isotopes Archives Dynamic Periodic Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes For stable elements, there is usually a variety of stable isotopes. 24 mg (23.985) with 78.99% abundance, 25 mg (24.986) with 10.00% abundance, and a. There are 19 radioisotopes that have been. The symbol for an atom. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the. The weighted average of. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From www.buyisotope.com
Magnesium25, Magnesium25 Isotope, Enriched Magnesium25 Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the. The weighted average of the individual isotopes is the atomic mass quoted on the periodic table. 24 mg (23.985) with 78.99% abundance, 25 mg (24.986) with 10.00% abundance, and a. 78.70% of all magnesium atoms have an atomic weight of 23.985. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From www.abhayjere.com
Calculating Average Atomic Mass Worksheet Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes Magnesium has three naturally occurring isotopes: Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the. There are 19 radioisotopes that have been. 78.70% of all magnesium atoms have an atomic weight of 23.985 amu, 10.13% have an. The magnesium isotope has a mass of 24 and a charge of +2,. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From pediaa.com
Difference Between Atomic Number and Mass Number Definition Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes Magnesium (mg) has three significant natural isotopes: There are 19 radioisotopes that have been. 78.70% of all magnesium atoms have an atomic weight of 23.985 amu, 10.13% have an. Magnesium has three naturally occurring isotopes: The magnesium isotope has a mass of 24 and a charge of +2, so it is written as 24 mg 2 +. Because most elements. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From wisc.pb.unizin.org
Isotopes, Atomic Mass, and Mass Spectrometry (M2Q3) UWMadison Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes The magnesium isotope has a mass of 24 and a charge of +2, so it is written as 24 mg 2 +. 24 mg (23.985) with 78.99% abundance, 25 mg (24.986) with 10.00% abundance, and a. 78.70% of all magnesium atoms have an atomic weight of 23.985 amu, 10.13% have an. Magnesium (mg) has three significant natural isotopes: The weighted. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From fr.depositphotos.com
Modèle d'atome de magnésium image vectorielle par Lukaves Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes The symbol for an atom. 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: The magnesium isotope has a mass of 24 and a charge of +2, so it is written as 24 mg 2 +. For stable elements, there is usually a variety of stable isotopes. The weighted average of the individual isotopes is the atomic mass. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From www.chegg.com
Solved Magnesium has three naturally occurring isotopes Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes There are 19 radioisotopes that have been. Magnesium has three naturally occurring isotopes: The weighted average of the individual isotopes is the atomic mass quoted on the periodic table. The symbol for an atom. Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the. For stable elements, there is usually a. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From es.slideshare.net
Final Review Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes Magnesium has three naturally occurring isotopes: 78.70% of all magnesium atoms have an atomic weight of 23.985 amu, 10.13% have an. There are 19 radioisotopes that have been. Because most elements exist as mixtures of several stable isotopes, the atomic mass of an element is defined as the weighted average of the masses of the isotopes. 46 rows magnesium (12. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From www.pinterest.co.uk
Magnesium, atomic structure Stock Image C018/3693 Science Photo Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes There are 19 radioisotopes that have been. 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: The weighted average of the individual isotopes is the atomic mass quoted on the periodic table. For stable elements, there is usually a variety of stable isotopes. The symbol for an atom. Magnesium has three naturally occurring isotopes: Isotopes are nuclides that. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From general.chemistrysteps.com
How To Calculate The Average Atomic Mass Chemistry Steps Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes The weighted average of the individual isotopes is the atomic mass quoted on the periodic table. 78.70% of all magnesium atoms have an atomic weight of 23.985 amu, 10.13% have an. Magnesium has three naturally occurring isotopes: Magnesium (mg) has three significant natural isotopes: For stable elements, there is usually a variety of stable isotopes. 46 rows magnesium (12 mg). Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From kennymeowbranch.blogspot.com
Relative Atomic Mass of Magnesium Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes 78.70% of all magnesium atoms have an atomic weight of 23.985 amu, 10.13% have an. Magnesium (mg) has three significant natural isotopes: Isotopes are nuclides that have the same atomic number and are therefore the same element, but differ in the. 46 rows magnesium (12 mg) naturally occurs in three stable isotopes: The magnesium isotope has a mass of 24. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From www.myxxgirl.com
Atom Diagram Of Magnesium Diagram To Show Ionic Bonding In Magnesium Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes The weighted average of the individual isotopes is the atomic mass quoted on the periodic table. The magnesium isotope has a mass of 24 and a charge of +2, so it is written as 24 mg 2 +. 24 mg (23.985) with 78.99% abundance, 25 mg (24.986) with 10.00% abundance, and a. Because most elements exist as mixtures of several. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From noradesnhharper.blogspot.com
Relative Atomic Mass of Magnesium Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes Magnesium has three naturally occurring isotopes: 24 mg (23.985) with 78.99% abundance, 25 mg (24.986) with 10.00% abundance, and a. Magnesium (mg) has three significant natural isotopes: The symbol for an atom. The weighted average of the individual isotopes is the atomic mass quoted on the periodic table. Isotopes are nuclides that have the same atomic number and are therefore. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.
From www.youtube.com
How to Find the Mass of One Atom of Magnesium (Mg) YouTube Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes Magnesium has three naturally occurring isotopes: The symbol for an atom. There are 19 radioisotopes that have been. Magnesium (mg) has three significant natural isotopes: The weighted average of the individual isotopes is the atomic mass quoted on the periodic table. For stable elements, there is usually a variety of stable isotopes. The magnesium isotope has a mass of 24. Magnesium Has An Atomic Mass Of 24.3. There Are Two Isotopes.