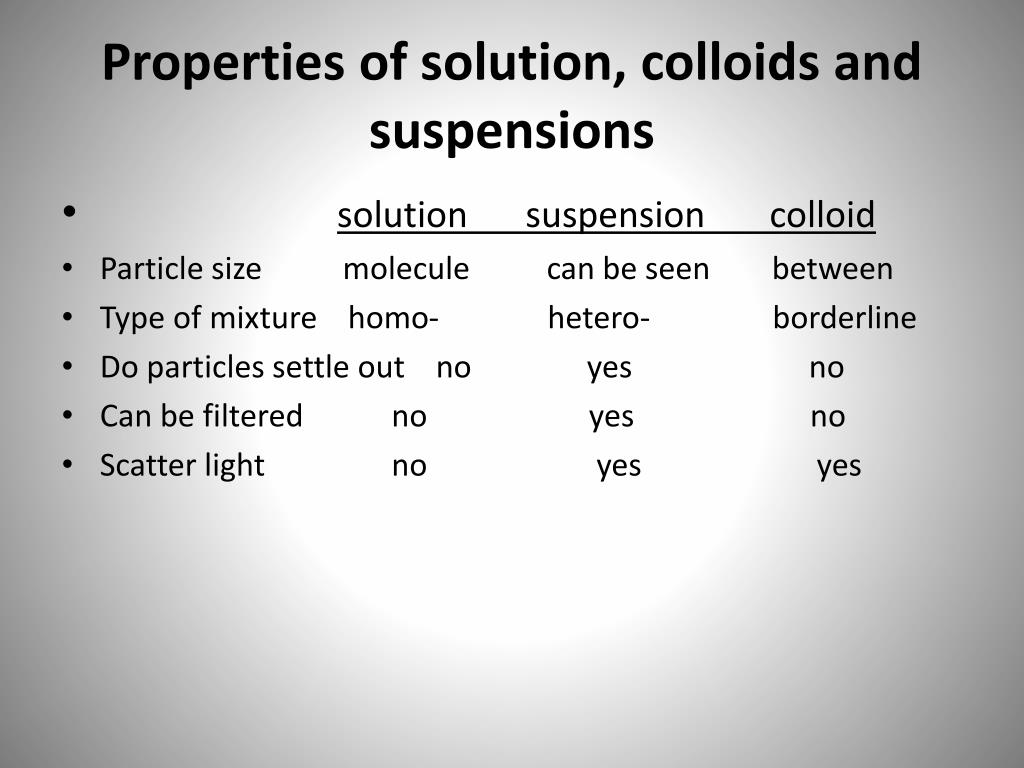
Suspension Vs Colloid Particle Size . Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. The components of a colloid are the dispersed. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. The particles in a colloid are larger than individual molecules but smaller than those found in suspensions. In contrast, a solution is a homogeneous mixture where the solute dissolves into the solvent. Colloid particles are not visible and they don’t settle out. A suspension is a heterogeneous mixture of two substances where the solute particles do not dissolve but remain dispersed through the bulk of the solvent. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other substance. Solutions, colloids, and suspensions differ in their particle size, homogeneity, and stability. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; These particles can be solid,. Colloid particles are much smaller than suspension particles. Components of a suspension can be evenly distributed by mechanical means, like by shaking the contents but the components will eventually settle out. The main difference between colloid and suspension lies in the size of particles. The dispersed particles separate from the dispersing phase on standing.
from www.slideserve.com
Colloid particles are much smaller than suspension particles. Colloid particles are not visible and they don’t settle out. These particles can be solid,. Components of a suspension can be evenly distributed by mechanical means, like by shaking the contents but the components will eventually settle out. A suspension is a heterogeneous mixture of two substances where the solute particles do not dissolve but remain dispersed through the bulk of the solvent. The dispersed particles separate from the dispersing phase on standing. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; The particles in suspensions are larger than those found in solutions. The main difference between colloid and suspension lies in the size of particles.
PPT Solutions and pH PowerPoint Presentation, free download ID5498133
Suspension Vs Colloid Particle Size Solutions, colloids, and suspensions differ in their particle size, homogeneity, and stability. The dispersed particles separate from the dispersing phase on standing. The particles in suspensions are larger than those found in solutions. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. Solutions, colloids, and suspensions differ in their particle size, homogeneity, and stability. Colloid particles are much smaller than suspension particles. The particles in a colloid are larger than individual molecules but smaller than those found in suspensions. These particles can be solid,. Colloid particles are not visible and they don’t settle out. Components of a suspension can be evenly distributed by mechanical means, like by shaking the contents but the components will eventually settle out. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; The components of a colloid are the dispersed. The main difference between colloid and suspension lies in the size of particles. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A suspension is a heterogeneous mixture of two substances where the solute particles do not dissolve but remain dispersed through the bulk of the solvent. In contrast, a solution is a homogeneous mixture where the solute dissolves into the solvent.
From www.slideserve.com
PPT Matter Properties & Change PowerPoint Presentation, free Suspension Vs Colloid Particle Size The dispersed particles separate from the dispersing phase on standing. Colloid particles are much smaller than suspension particles. The main difference between colloid and suspension lies in the size of particles. The components of a colloid are the dispersed. The particles in a colloid are larger than individual molecules but smaller than those found in suspensions. The particles in suspensions. Suspension Vs Colloid Particle Size.
From www.slideserve.com
PPT SOLUTION CHEMISTRY PowerPoint Presentation, free download ID Suspension Vs Colloid Particle Size The dispersed particles separate from the dispersing phase on standing. The particles in a colloid are larger than individual molecules but smaller than those found in suspensions. A suspension is a heterogeneous mixture of two substances where the solute particles do not dissolve but remain dispersed through the bulk of the solvent. A suspension is a heterogeneous mixture of particles. Suspension Vs Colloid Particle Size.
From askanydifference.com
Colloid vs Suspension Difference and Comparison Suspension Vs Colloid Particle Size The components of a colloid are the dispersed. These particles can be solid,. Colloid particles are much smaller than suspension particles. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. A suspension is a heterogeneous mixture of two substances where the solute particles do not dissolve. Suspension Vs Colloid Particle Size.
From www.vecteezy.com
True Solution, Colloid solution and Suspension three different types of Suspension Vs Colloid Particle Size Colloid particles are not visible and they don’t settle out. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. The main difference between colloid and suspension lies in the size of particles. Components of a suspension can be evenly distributed by mechanical means, like by shaking the contents but the components will eventually. Suspension Vs Colloid Particle Size.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Suspension Vs Colloid Particle Size Colloid particles are much smaller than suspension particles. The components of a colloid are the dispersed. The particles in suspensions are larger than those found in solutions. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. Components of a suspension can be evenly distributed by mechanical means, like by shaking the contents but. Suspension Vs Colloid Particle Size.
From www.researchgate.net
Particle Size Diameter of Reported Colloids Download Table Suspension Vs Colloid Particle Size Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. Colloid particles are much smaller than suspension particles. A suspension is a heterogeneous mixture of two substances where the solute particles do not dissolve but remain dispersed through the bulk of the solvent. The particles in suspensions are larger than those found in solutions.. Suspension Vs Colloid Particle Size.
From www.nagwa.com
Vidéo de la leçon Colloïdes et les suspensions Nagwa Suspension Vs Colloid Particle Size A suspension is a heterogeneous mixture of two substances where the solute particles do not dissolve but remain dispersed through the bulk of the solvent. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. Colloid particles are not visible and they don’t settle out. The particles. Suspension Vs Colloid Particle Size.
From www.sliderbase.com
Colloids and Suspensions Presentation Chemistry Suspension Vs Colloid Particle Size The particles in suspensions are larger than those found in solutions. Colloid particles are much smaller than suspension particles. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other substance. The main difference between colloid and suspension lies in the size of particles. Components of a suspension can be evenly. Suspension Vs Colloid Particle Size.
From www.slideserve.com
PPT 2 PowerPoint Presentation, free download ID6415741 Suspension Vs Colloid Particle Size The particles in a colloid are larger than individual molecules but smaller than those found in suspensions. The main difference between colloid and suspension lies in the size of particles. Components of a suspension can be evenly distributed by mechanical means, like by shaking the contents but the components will eventually settle out. The components of a colloid are the. Suspension Vs Colloid Particle Size.
From www.geeksforgeeks.org
What is a Suspension? Suspension Vs Colloid Particle Size A suspension is a heterogeneous mixture of two substances where the solute particles do not dissolve but remain dispersed through the bulk of the solvent. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. Solutions, colloids, and suspensions differ in their particle size, homogeneity, and stability. These particles can be solid,. Colloid particles. Suspension Vs Colloid Particle Size.
From quizizz.com
Solution, Colloid and Suspension Science Quizizz Suspension Vs Colloid Particle Size Components of a suspension can be evenly distributed by mechanical means, like by shaking the contents but the components will eventually settle out. Solutions, colloids, and suspensions differ in their particle size, homogeneity, and stability. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; Colloid particles are not visible and they don’t settle. Suspension Vs Colloid Particle Size.
From www.askdifference.com
Colloid vs. Suspension — What’s the Difference? Suspension Vs Colloid Particle Size The particles in a colloid are larger than individual molecules but smaller than those found in suspensions. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other substance. The components of a colloid are. Suspension Vs Colloid Particle Size.
From www.geeksforgeeks.org
Suspension(Chemistry) Definition, Properties, Examples, and FAQs Suspension Vs Colloid Particle Size The components of a colloid are the dispersed. Solutions, colloids, and suspensions differ in their particle size, homogeneity, and stability. Colloid particles are much smaller than suspension particles. The particles in a colloid are larger than individual molecules but smaller than those found in suspensions. Components of a suspension can be evenly distributed by mechanical means, like by shaking the. Suspension Vs Colloid Particle Size.
From www.sliderbase.com
Suspensions and Colloids Presentation Chemistry Suspension Vs Colloid Particle Size Solutions, colloids, and suspensions differ in their particle size, homogeneity, and stability. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other substance. The main difference between colloid and suspension lies in the size. Suspension Vs Colloid Particle Size.
From slideplayer.com
Solutions, Colloids, and Suspensions ppt download Suspension Vs Colloid Particle Size Components of a suspension can be evenly distributed by mechanical means, like by shaking the contents but the components will eventually settle out. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. A suspension is a heterogeneous mixture of two substances where the solute particles do not dissolve but remain dispersed through the. Suspension Vs Colloid Particle Size.
From gioglbtpv.blob.core.windows.net
Suspension And Colloid And Solution at Jason Vasquez blog Suspension Vs Colloid Particle Size These particles can be solid,. The components of a colloid are the dispersed. Components of a suspension can be evenly distributed by mechanical means, like by shaking the contents but the components will eventually settle out. A suspension is a heterogeneous mixture of two substances where the solute particles do not dissolve but remain dispersed through the bulk of the. Suspension Vs Colloid Particle Size.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Suspension Vs Colloid Particle Size Colloid particles are not visible and they don’t settle out. In contrast, a solution is a homogeneous mixture where the solute dissolves into the solvent. The dispersed particles separate from the dispersing phase on standing. These particles can be solid,. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle. Suspension Vs Colloid Particle Size.
From www.shutterstock.com
218 vectores de Colloid Vectores, imágenes y arte vectorial de stock Suspension Vs Colloid Particle Size The main difference between colloid and suspension lies in the size of particles. A suspension is a heterogeneous mixture of two substances where the solute particles do not dissolve but remain dispersed through the bulk of the solvent. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. The components of a colloid are. Suspension Vs Colloid Particle Size.
From pediaa.com
Difference Between Colloid and Solution Definition, Properties Suspension Vs Colloid Particle Size The main difference between colloid and suspension lies in the size of particles. The dispersed particles separate from the dispersing phase on standing. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other substance. In contrast, a solution is a homogeneous mixture where the solute dissolves into the solvent. Solutions,. Suspension Vs Colloid Particle Size.
From www.difference.wiki
Colloid vs. Suspension What’s the Difference? Suspension Vs Colloid Particle Size The particles in a colloid are larger than individual molecules but smaller than those found in suspensions. Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. These particles can be solid,. Solutions, colloids, and suspensions differ in their particle size, homogeneity, and stability. The components of. Suspension Vs Colloid Particle Size.
From chemwiki.ucdavis.edu
Figure 1 Examples of a stable and of an unstable colloidal dispersion Suspension Vs Colloid Particle Size In contrast, a solution is a homogeneous mixture where the solute dissolves into the solvent. Solutions, colloids, and suspensions differ in their particle size, homogeneity, and stability. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. The particles in suspensions are larger than those found in solutions. Colloid particles are much smaller than. Suspension Vs Colloid Particle Size.
From www.toppr.com
(k) Differentiate between solution, suspension and colloids on the Suspension Vs Colloid Particle Size In contrast, a solution is a homogeneous mixture where the solute dissolves into the solvent. A suspension is a heterogeneous mixture of two substances where the solute particles do not dissolve but remain dispersed through the bulk of the solvent. These particles can be solid,. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second. Suspension Vs Colloid Particle Size.
From www.differencebetween.com
Difference Between Suspension and Colloid Compare the Difference Suspension Vs Colloid Particle Size In contrast, a solution is a homogeneous mixture where the solute dissolves into the solvent. A suspension is a heterogeneous mixture of two substances where the solute particles do not dissolve but remain dispersed through the bulk of the solvent. Components of a suspension can be evenly distributed by mechanical means, like by shaking the contents but the components will. Suspension Vs Colloid Particle Size.
From www.slideserve.com
PPT Solutions and pH PowerPoint Presentation, free download ID5498133 Suspension Vs Colloid Particle Size Because the dispersed particles of a colloid are not as large as those of a suspension, they do not settle out upon standing. The particles in a colloid are larger than individual molecules but smaller than those found in suspensions. A suspension is a heterogeneous mixture of two substances where the solute particles do not dissolve but remain dispersed through. Suspension Vs Colloid Particle Size.
From slideplayer.com
(solids) Solutions and Other Mixtures ppt download Suspension Vs Colloid Particle Size A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other substance. Solutions, colloids, and suspensions differ in their particle size, homogeneity, and stability. The particles in a colloid are larger than individual molecules but smaller than those found in suspensions. The components of a colloid are the dispersed. The main. Suspension Vs Colloid Particle Size.
From pediaa.com
Difference Between Colloid and Suspension Definition, Properties Suspension Vs Colloid Particle Size The particles in a colloid are larger than individual molecules but smaller than those found in suspensions. A suspension is a heterogeneous mixture of two substances where the solute particles do not dissolve but remain dispersed through the bulk of the solvent. The dispersed particles separate from the dispersing phase on standing. A colloid is a mixture in which one. Suspension Vs Colloid Particle Size.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Suspension Vs Colloid Particle Size In contrast, a solution is a homogeneous mixture where the solute dissolves into the solvent. The main difference between colloid and suspension lies in the size of particles. A suspension is a heterogeneous mixture of two substances where the solute particles do not dissolve but remain dispersed through the bulk of the solvent. A colloid is a mixture in which. Suspension Vs Colloid Particle Size.
From byjus.com
Suspensions & Colloids Difference Between Colloid & SuspensionByju's Suspension Vs Colloid Particle Size Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. These particles can be solid,. The dispersed particles separate from the dispersing phase on standing. The components of a colloid are the dispersed. Components of a suspension can be evenly distributed by mechanical means, like by shaking the contents but the components will eventually. Suspension Vs Colloid Particle Size.
From scsscience9.blogspot.com
Is Matter Around Us Pure? Blog 6 Suspension Vs Colloid Particle Size A suspension is a heterogeneous mixture of two substances where the solute particles do not dissolve but remain dispersed through the bulk of the solvent. The components of a colloid are the dispersed. Components of a suspension can be evenly distributed by mechanical means, like by shaking the contents but the components will eventually settle out. Colloid particles are not. Suspension Vs Colloid Particle Size.
From www.differencebetween.net
Difference Between Colloid and Suspension Difference Between Suspension Vs Colloid Particle Size The dispersed particles separate from the dispersing phase on standing. A suspension is a heterogeneous mixture of particles of one substance distributed throughout a second phase; Solutions, colloids, and suspensions differ in their particle size, homogeneity, and stability. The particles in a colloid are larger than individual molecules but smaller than those found in suspensions. Components of a suspension can. Suspension Vs Colloid Particle Size.
From www.slideserve.com
PPT Chapter 12 SOLUTIONS PowerPoint Presentation, free download ID Suspension Vs Colloid Particle Size Colloid particles are not visible and they don’t settle out. A colloid is a mixture in which one of the soluble or insoluble particles is microscopically dispersed throughout the other substance. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. The dispersed particles separate from the dispersing phase on standing. Colloid particles are. Suspension Vs Colloid Particle Size.
From www.vecteezy.com
Different dispersion system, true and colloidal solution and suspension Suspension Vs Colloid Particle Size The dispersed particles separate from the dispersing phase on standing. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. The components of a colloid are the dispersed. Components of a suspension can be evenly distributed by mechanical means, like by shaking the contents but the components will eventually settle out. Colloid particles are. Suspension Vs Colloid Particle Size.
From wiki.anton-paar.com
The influence of particles on suspension rheology Anton Paar Wiki Suspension Vs Colloid Particle Size A suspension is a heterogeneous mixture of two substances where the solute particles do not dissolve but remain dispersed through the bulk of the solvent. The main difference between colloid and suspension lies in the size of particles. The components of a colloid are the dispersed. These particles can be solid,. In contrast, a solution is a homogeneous mixture where. Suspension Vs Colloid Particle Size.
From www.animalia-life.club
Examples Of Colloids Mixtures Suspension Vs Colloid Particle Size The particles in suspensions are larger than those found in solutions. The main difference between colloid and suspension lies in the size of particles. The components of a colloid are the dispersed. Colloid particles are much smaller than suspension particles. Colloid particles are not visible and they don’t settle out. A colloid is a mixture in which one of the. Suspension Vs Colloid Particle Size.
From www.majordifferences.com
Difference Between True Solutions, Colloidal solution and Suspension Suspension Vs Colloid Particle Size The particles in suspensions are larger than those found in solutions. Both suspensions and colloids are heterogeneous mixtures, but the particle size in a suspension is larger. The particles in a colloid are larger than individual molecules but smaller than those found in suspensions. Because the dispersed particles of a colloid are not as large as those of a suspension,. Suspension Vs Colloid Particle Size.