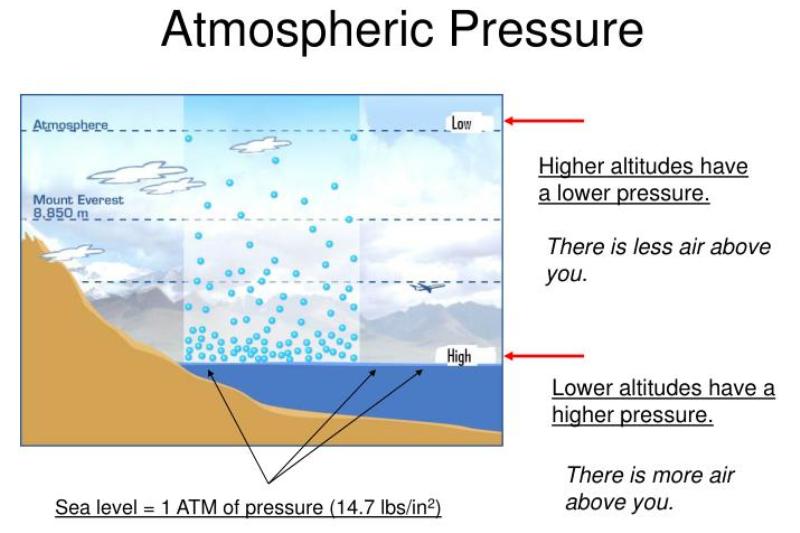
What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid . The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals. The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid. Normally when we boil a liquid, we do so at atmospheric pressure. Because atmospheric pressure can change based on location, the boiling. This is the pressure exerted by the weight of the air molecules. The boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure. The boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the temperature at which its vapor. When the vapour pressure equals atmospheric pressure, the liquid boils. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. As elevation increases, atmospheric pressure decreases because air is less dense at higher. The boiling point of a liquid is directly affected by atmospheric pressure. The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure.
from studiousguy.com
When the vapour pressure equals atmospheric pressure, the liquid boils. The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid. The boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the temperature at which its vapor. This is the pressure exerted by the weight of the air molecules. The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals. Normally when we boil a liquid, we do so at atmospheric pressure. The boiling point of a liquid is directly affected by atmospheric pressure. As elevation increases, atmospheric pressure decreases because air is less dense at higher. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is.
Boiling Point Examples in Everyday Life StudiousGuy
What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals. This is the pressure exerted by the weight of the air molecules. Normally when we boil a liquid, we do so at atmospheric pressure. The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid. The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. When the vapour pressure equals atmospheric pressure, the liquid boils. As elevation increases, atmospheric pressure decreases because air is less dense at higher. The boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the temperature at which its vapor. Because atmospheric pressure can change based on location, the boiling. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals. The boiling point of a liquid is directly affected by atmospheric pressure. The boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is.
From www.vrogue.co
How Do Atmospheric Pressure And Elevation Affect Boil vrogue.co What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid. As elevation increases, atmospheric pressure decreases because air is less dense at higher. The boiling point of a liquid is the temperature. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From mavink.com
Water Vapour Pressure Chart What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid The boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the temperature at which its vapor. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. When the. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid The boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure. The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. The boiling point of a liquid is directly affected by atmospheric pressure. The normal boiling point (also called the atmospheric boiling point or the. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From physicsexperiments.eu
Dependence of Boiling Point of Water on Pressure — Collection of What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid The boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure. The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. Because atmospheric pressure can change based on location, the boiling. The boiling point of a liquid is directly affected by atmospheric pressure. The boiling. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From kenkidryer.com
Saturation temperature (boiling point) KENKI DRYER What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. When the vapour pressure equals atmospheric pressure, the liquid boils. The boiling point is reached when the vapor pressure. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From mr.kentchemistry.com
Determine Boiling Point from Vapor Pressure What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. As elevation increases, atmospheric pressure decreases because air is less dense at higher. The boiling point of a liquid is directly affected by atmospheric pressure. The boiling point is reached when the vapor pressure of a liquid matches the atmospheric. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From sciencenotes.org
Boiling Point of Water What Temperature Does Water Boil? What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid The boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the temperature at which its vapor. The boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure. As elevation increases, atmospheric pressure decreases because. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From www.fiziklah.com
Suhu dan Altitud Fiziklah! What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals. Because atmospheric pressure can change based on location, the boiling. The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From guides.hostos.cuny.edu
Chapter 3 Solids and Liquids CHE 110 Introduction to Chemistry What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid When the vapour pressure equals atmospheric pressure, the liquid boils. The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid. The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. Normally when we boil a liquid, we do so at atmospheric pressure.. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From exomkbouq.blob.core.windows.net
What Is Boiling Point Elevation Constant at Ronnie Moore blog What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid Normally when we boil a liquid, we do so at atmospheric pressure. As elevation increases, atmospheric pressure decreases because air is less dense at higher. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals. The normal boiling. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From www.peoi.org
Chapter 10 Section C Properties of Liquids What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals. The boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From www.myxxgirl.com
Boiling Point Vs Pressure Chart My XXX Hot Girl What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. The boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. When the vapour. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From collinewelshs.blob.core.windows.net
Pressure Cooker Boiling Point Of Water at collinewelshs blog What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid The boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the temperature at which its vapor. The boiling point of a liquid is directly affected by atmospheric pressure. The boiling point is reached when the vapor pressure of a. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From sciencenotes.org
Vapor Pressure Definition and How to Calculate It What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid The boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure. The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. This is the pressure exerted by the weight of the air molecules. The boiling point of a liquid is directly affected by atmospheric pressure.. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From www.youtube.com
Introduction to Pressure Force & Area, Units, Atmospheric Gases What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid Normally when we boil a liquid, we do so at atmospheric pressure. The boiling point of a liquid is directly affected by atmospheric pressure. When the vapour pressure equals atmospheric pressure, the liquid boils. Because atmospheric pressure can change based on location, the boiling. The normal boiling point is the temperature at which the vapor pressure of the liquid is. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From www.alamy.com
Atmospheric pressure alters the boiling point of water Stock Photo Alamy What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals. Because atmospheric pressure can change based on location, the boiling.. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From www.peoi.org
Chapter 10 Section C Properties of Liquids What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid Because atmospheric pressure can change based on location, the boiling. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals. As elevation increases, atmospheric pressure decreases because air is less dense at higher. This is the pressure exerted. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From pediaa.com
Difference Between Vapor Pressure and Boiling Point Definition What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid Because atmospheric pressure can change based on location, the boiling. The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid. This is the pressure exerted by the weight of the air molecules. The boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure. The boiling point of. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From surfguppy.com
How does Atmospheric Pressure Affect Boiling Point What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid The boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure. Because atmospheric pressure can change based on location, the boiling. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals. The boiling. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From www.wave3.com
Behind the Forecast Outwitting the weather when baking What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid When the vapour pressure equals atmospheric pressure, the liquid boils. The boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals. If this. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From studiousguy.com
Boiling Point Examples in Everyday Life StudiousGuy What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid The boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the temperature at which its vapor. This is the pressure exerted by the weight of the air molecules. The boiling point of a liquid depends on temperature, atmospheric pressure,. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From youtube.com
Evaporation, Vapor Pressure and Boiling YouTube What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid. This is the pressure exerted by the weight of the air molecules. The boiling point of a liquid is directly affected. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From mungfali.com
Vapor Pressure And Boiling Point Relationship What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid The boiling point of a liquid is directly affected by atmospheric pressure. The boiling point of a liquid depends on temperature, atmospheric pressure, and the vapor pressure of the liquid. As elevation increases, atmospheric pressure decreases because air is less dense at higher. Normally when we boil a liquid, we do so at atmospheric pressure. Because atmospheric pressure can change. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From www.globalspec.com
Chapter 3 Physical Properties of Fluids Vapor Pressure and Boiling What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. The boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure. The boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From chemistryskills.com
Definition and Explanation of Boiling Point Chemistry Skills What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid Normally when we boil a liquid, we do so at atmospheric pressure. As elevation increases, atmospheric pressure decreases because air is less dense at higher. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the liquid boils is. The boiling point of a liquid is directly affected by atmospheric pressure. The normal. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From www.dreamstime.com
Boiling and Evaporation, Freezing and Melting Points of Water Stock What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid The boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure. Normally when we boil a liquid, we do so at atmospheric pressure. The boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of a liquid is the. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From surfguppy.com
How does Atmospheric Pressure Affect Boiling Point What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid The boiling point is reached when the vapor pressure of a liquid matches the atmospheric pressure. The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals. The boiling point of a liquid is the temperature at which its. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From www.pinterest.es
Atmospheric pressure and boiling point of water table Boiling Point What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid Normally when we boil a liquid, we do so at atmospheric pressure. When the vapour pressure equals atmospheric pressure, the liquid boils. The normal boiling point is the temperature at which the vapor pressure of the liquid is equal to standard pressure. If this pressure is the standard pressure of 1 atm (101.3 kpa), then the temperature at which the. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.
From www.youtube.com
Vapor Pressure and Boiling YouTube What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals. The boiling point of a liquid is the temperature at which its vapor pressure is equal to the pressure of the gas above it.the normal boiling point of. What Is The Relationship Between Atmospheric Pressure And Boiling Point Of A Liquid.