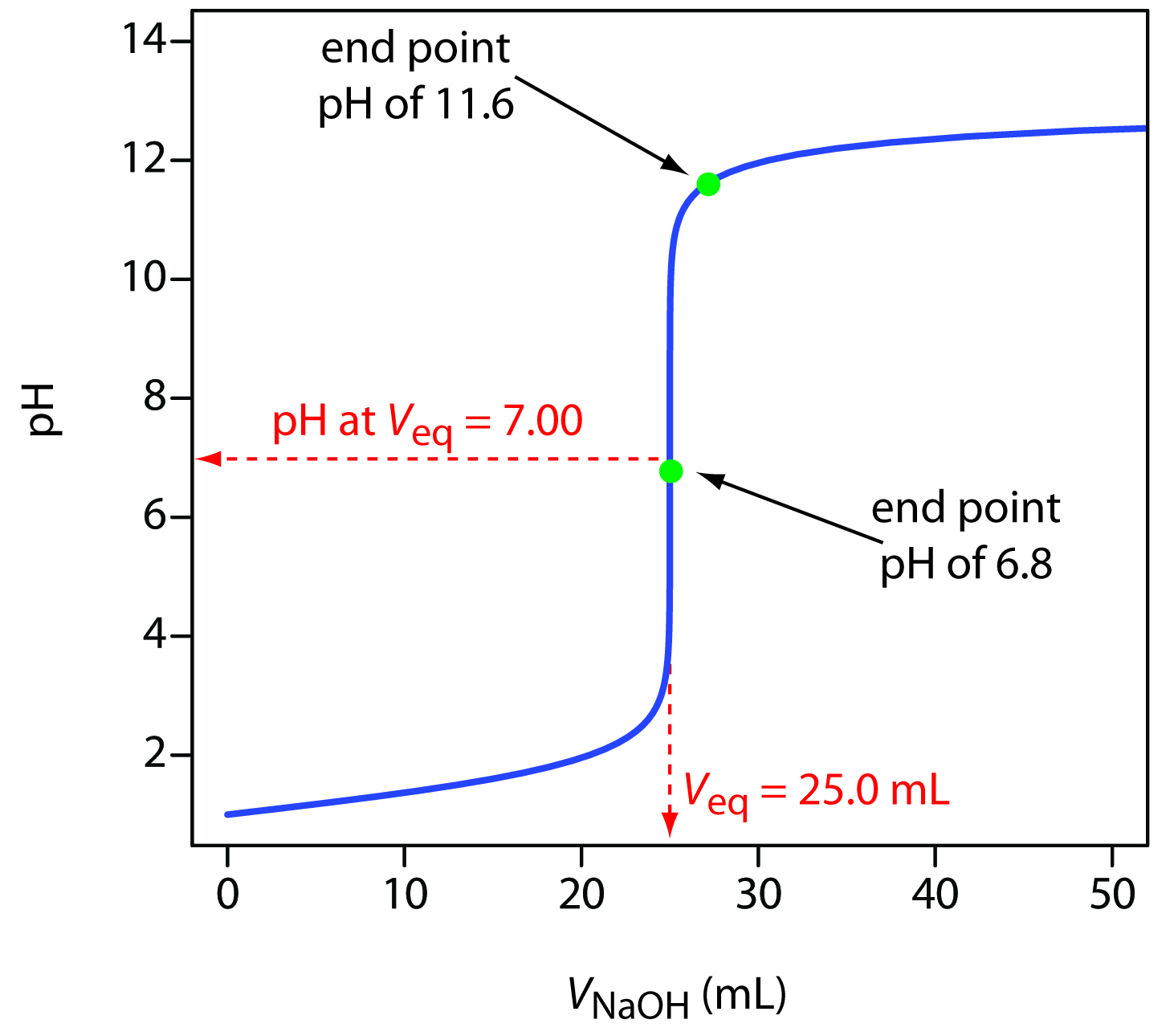
Half Titration Meaning . It is based on the. titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. in a titration this is known as half equivalence or half titer, which is the volume required to titrate off half of the. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. See titration curves for acids and bases. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its.
from chem.libretexts.org
the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. in a titration this is known as half equivalence or half titer, which is the volume required to titrate off half of the. titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. It is based on the. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. See titration curves for acids and bases.
9.1 Overview of Titrimetry Chemistry LibreTexts
Half Titration Meaning See titration curves for acids and bases. in a titration this is known as half equivalence or half titer, which is the volume required to titrate off half of the. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. See titration curves for acids and bases. the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its. It is based on the. titration is an analytical chemistry technique used to find the concentration of an unknown acid or base.
From thechemistrynotes.com
Titration Definition, 4 Types, Procedure Half Titration Meaning See titration curves for acids and bases. in a titration this is known as half equivalence or half titer, which is the volume required to titrate off half of the. It is based on the. the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. sketch out a plot. Half Titration Meaning.
From gamesmartz.com
Titration Definition & Image GameSmartz Half Titration Meaning the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. See titration curves for acids and bases. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its. titration is an analytical chemistry. Half Titration Meaning.
From chem.libretexts.org
Titration of a Weak Base with a Strong Acid Chemistry LibreTexts Half Titration Meaning titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. It is based on the. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or. Half Titration Meaning.
From exoqetgcr.blob.core.windows.net
Titration Experiment Meaning at Rosa Baldwin blog Half Titration Meaning It is based on the. in a titration this is known as half equivalence or half titer, which is the volume required to titrate off half of the. titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. a titration is an experiment where a volume of a solution of. Half Titration Meaning.
From www.slideserve.com
PPT Ch 16 Redox Titrations PowerPoint Presentation, free download Half Titration Meaning It is based on the. the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. a titration is an experiment where a volume of a solution. Half Titration Meaning.
From www.slideserve.com
PPT Chem. 31 2/3 Lecture PowerPoint Presentation, free download Half Titration Meaning See titration curves for acids and bases. in a titration this is known as half equivalence or half titer, which is the volume required to titrate off half of the. It is based on the. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in. Half Titration Meaning.
From www.vrogue.co
Ph Indicators Titration Curves Teaching Resources vrogue.co Half Titration Meaning the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. See titration curves for acids and bases. in a titration this is known as half equivalence or half titer, which is the volume required to titrate off half of the. sketch out a plot representing the titration of a. Half Titration Meaning.
From studylib.net
Half Titration Peroxide The Cervantes Chemistry Page Half Titration Meaning See titration curves for acids and bases. in a titration this is known as half equivalence or half titer, which is the volume required to titrate off half of the. It is based on the. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by.. Half Titration Meaning.
From cextumpt.blob.core.windows.net
Titration Definition Chemistry Class 11 at Donald Chapman blog Half Titration Meaning See titration curves for acids and bases. titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. in a titration this is known as half equivalence or half titer, which is the volume required to titrate off half of the. the first example involves a strong acid titration that requires. Half Titration Meaning.
From present5.com
Titration and AcidBase Neutralization LEARNING OBJECTIVES 11.2.2.7 Half Titration Meaning titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its. It is based on the. the first example involves a strong acid titration. Half Titration Meaning.
From www.youtube.com
titration part1 definition YouTube Half Titration Meaning in a titration this is known as half equivalence or half titer, which is the volume required to titrate off half of the. the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. titration is an analytical chemistry technique used to find the concentration of an unknown acid or. Half Titration Meaning.
From cextumpt.blob.core.windows.net
Titration Definition Chemistry Class 11 at Donald Chapman blog Half Titration Meaning a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its. See titration curves for acids and bases. It is based on the. titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. in. Half Titration Meaning.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Half Titration Meaning It is based on the. in a titration this is known as half equivalence or half titer, which is the volume required to titrate off half of the. titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. See titration curves for acids and bases. sketch out a plot representing. Half Titration Meaning.
From mavink.com
Titration Labeled Half Titration Meaning titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. in a titration this is known as half equivalence or half titer, which is the volume required to titrate off half of the. It is based on the. the first example involves a strong acid titration that requires only stoichiometric. Half Titration Meaning.
From www.youtube.com
Chapter 17 second half titrations and Ksp YouTube Half Titration Meaning a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its. titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. See titration curves for acids and bases. sketch out a plot representing the. Half Titration Meaning.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Half Titration Meaning a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its. in a titration this is known as half equivalence or half titer, which is the volume required to titrate off half of the. See titration curves for acids and bases. . Half Titration Meaning.
From cehpcvvh.blob.core.windows.net
Titration Meaning In Chemistry at Donna Lewis blog Half Titration Meaning in a titration this is known as half equivalence or half titer, which is the volume required to titrate off half of the. It is based on the. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. titration is an analytical chemistry technique. Half Titration Meaning.
From dxozwvadj.blob.core.windows.net
Types Of Titration And Indicators Used at Lillie McIntosh blog Half Titration Meaning a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its. the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. sketch out a plot representing the titration of a weak monoprotic acid. Half Titration Meaning.
From dxomjwenv.blob.core.windows.net
Titration Half Equivalence Point at Karen Winkleman blog Half Titration Meaning a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its. It is based on the. titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. See titration curves for acids and bases. in. Half Titration Meaning.
From www.osmosis.org
Introduction to titrations Vídeo & Anatomía Osmosis Half Titration Meaning It is based on the. the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. titration is an analytical chemistry technique used to find the concentration. Half Titration Meaning.
From learningschoolmurgkerny1.z13.web.core.windows.net
How To Do Titrations In Chemistry Half Titration Meaning a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its. It is based on the. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. titration is. Half Titration Meaning.
From cehpcvvh.blob.core.windows.net
Titration Meaning In Chemistry at Donna Lewis blog Half Titration Meaning It is based on the. in a titration this is known as half equivalence or half titer, which is the volume required to titrate off half of the. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. See titration curves for acids and bases.. Half Titration Meaning.
From printablenemnserioeg.z22.web.core.windows.net
Titration Practical Questions And Answers Half Titration Meaning sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. It is based on the. in a titration this is known as half equivalence or half. Half Titration Meaning.
From www.youtube.com
fundamentals of volumetric analysis introduction to titration and Half Titration Meaning sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. in a titration this is known as half equivalence or half titer, which is the volume required. Half Titration Meaning.
From fernando-well-key.blogspot.com
How to Describe a Titration Curve Half Titration Meaning the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. It is based on the. in a titration this is known as half equivalence or half. Half Titration Meaning.
From www.youtube.com
Lecture 7 Titration Half Equivalence Point YouTube Half Titration Meaning See titration curves for acids and bases. It is based on the. the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its. . Half Titration Meaning.
From www.slideserve.com
PPT How to Interpret Titration Curves PowerPoint Presentation ID225155 Half Titration Meaning the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. in a titration this is known as half equivalence or half titer, which is the volume required to titrate off half of the. See titration curves for acids and bases. It is based on the. sketch out a plot. Half Titration Meaning.
From www.studypool.com
SOLUTION Chemistry acid base titration Studypool Half Titration Meaning the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution. Half Titration Meaning.
From www.slideserve.com
PPT Titration and pH Curves. PowerPoint Presentation, free download Half Titration Meaning in a titration this is known as half equivalence or half titer, which is the volume required to titrate off half of the. It is based on the. titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. a titration is an experiment where a volume of a solution of. Half Titration Meaning.
From slidetodoc.com
Titrations Titration A known concentration of base or Half Titration Meaning titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. the first example involves a strong acid titration that requires only stoichiometric calculations to derive the solution ph. sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated. Half Titration Meaning.
From sciencing.com
How to Find the Half Equivalence Point in a Titration Graph Sciencing Half Titration Meaning See titration curves for acids and bases. titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. in a titration this is known as half equivalence or half titer, which is the volume required to titrate off half of the. It is based on the. the first example involves a. Half Titration Meaning.
From www.writework.com
Titration of amino acids WriteWork Half Titration Meaning sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. It is based on the. a titration is an experiment where a volume of a solution of known concentration is added to a volume of another solution in order to determine its. in a. Half Titration Meaning.
From www.studypool.com
SOLUTION Titrations titration meaning Studypool Half Titration Meaning sketch out a plot representing the titration of a weak monoprotic acid by a strong base, or of a weak base titrated by. titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. See titration curves for acids and bases. in a titration this is known as half equivalence or. Half Titration Meaning.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Half Titration Meaning in a titration this is known as half equivalence or half titer, which is the volume required to titrate off half of the. titration is an analytical chemistry technique used to find the concentration of an unknown acid or base. It is based on the. a titration is an experiment where a volume of a solution of. Half Titration Meaning.