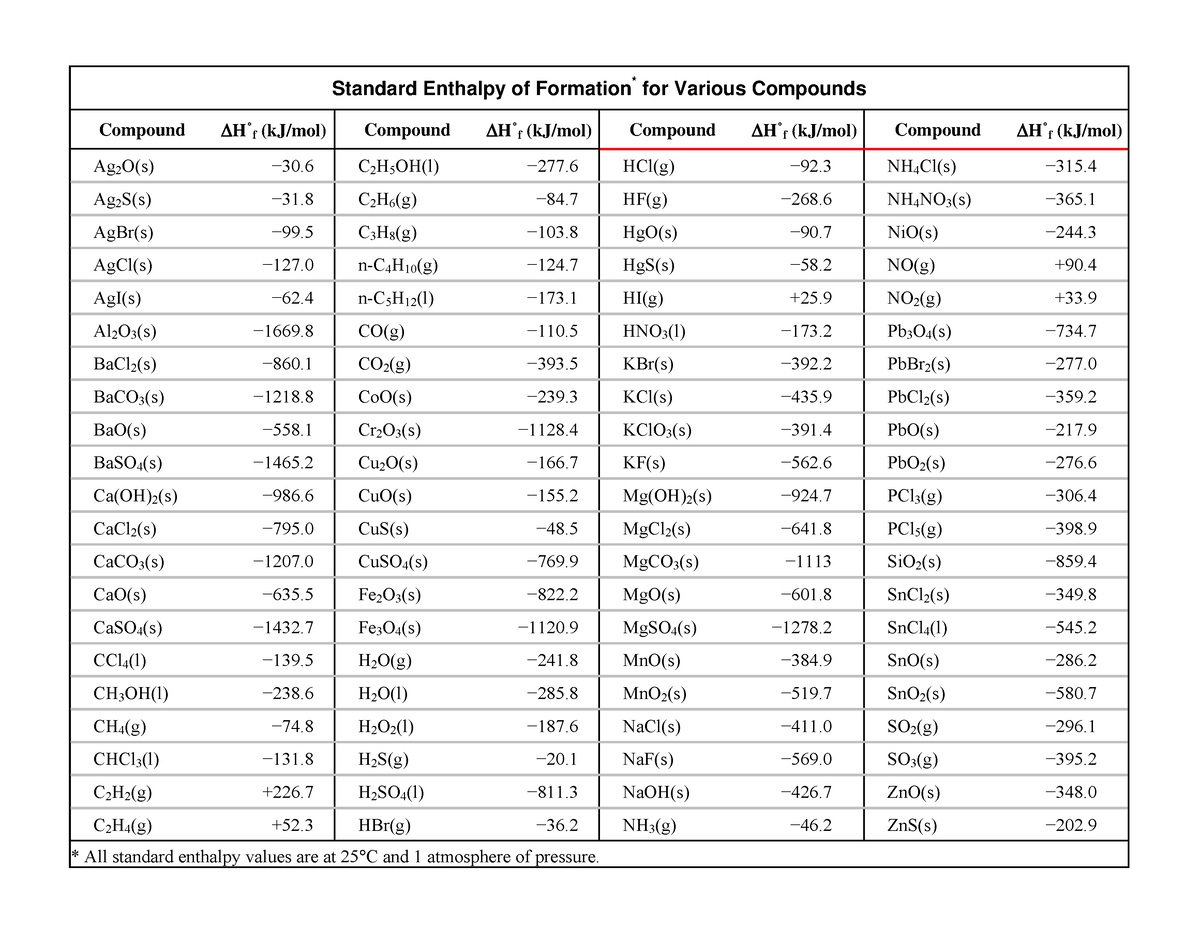
Standard Enthalpy Of Formation Difference . For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. Expressed differently, a molecule’s enthalpy of formation is the heat associated with forming it from its most basic components. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. For example, take the reaction forming hexane (c. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of.
from www.studocu.com
The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, take the reaction forming hexane (c. Expressed differently, a molecule’s enthalpy of formation is the heat associated with forming it from its most basic components. Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and.
Standard Enthalpy of Formation Table Standard Enthalpy of Formation
Standard Enthalpy Of Formation Difference The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. For example, take the reaction forming hexane (c. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when. The standard enthalpy of formation of any element in its standard state is zero by definition. Expressed differently, a molecule’s enthalpy of formation is the heat associated with forming it from its most basic components.
From www.w3schools.blog
Standard enthalpy of ionization W3schools Standard Enthalpy Of Formation Difference For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. Expressed differently, a molecule’s enthalpy of formation is the heat associated with forming it from its most basic components. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy. Standard Enthalpy Of Formation Difference.
From www.youtube.com
R1.2.3 / R1.2.4 Standard enthalpy change of formation (HL) YouTube Standard Enthalpy Of Formation Difference The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. For example, take the reaction. Standard Enthalpy Of Formation Difference.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Difference For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The standard enthalpy of formation of any element in its standard state. Standard Enthalpy Of Formation Difference.
From rayb78.github.io
Heat Of Formation Chart Standard Enthalpy Of Formation Difference For example, take the reaction forming hexane (c. Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. Expressed differently,. Standard Enthalpy Of Formation Difference.
From darkataxa.blogspot.com
Astounding Collections Of Heat Of Formation Table Photos Darkata Standard Enthalpy Of Formation Difference The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. Expressed differently, a molecule’s enthalpy of formation is the heat associated. Standard Enthalpy Of Formation Difference.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Enthalpy Of Formation Difference Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when. Expressed differently, a molecule’s enthalpy of formation is the heat associated with forming it from its most basic components. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. The standard enthalpy of formation is the enthalpy change. Standard Enthalpy Of Formation Difference.
From www.slideserve.com
PPT Chapter 5 Thermochemistry PowerPoint Presentation, free download Standard Enthalpy Of Formation Difference For example, take the reaction forming hexane (c. Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when. Expressed differently, a molecule’s enthalpy of formation is the heat associated with forming it from its most basic components. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change. Standard Enthalpy Of Formation Difference.
From www.slideshare.net
Standard enthalpy of formation Standard Enthalpy Of Formation Difference Expressed differently, a molecule’s enthalpy of formation is the heat associated with forming it from its most basic components. For example, take the reaction forming hexane (c. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. The standard. Standard Enthalpy Of Formation Difference.
From www.tessshebaylo.com
Balance The Following Chemical Equation And Calculate Standard Enthalpy Standard Enthalpy Of Formation Difference The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element in its standard state is. Standard Enthalpy Of Formation Difference.
From radenbayu33as.blogspot.com
Enthalpy Of Formation Of C3H8 Enthalpy Changes during Formation of Standard Enthalpy Of Formation Difference 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Expressed differently, a molecule’s enthalpy of formation is the heat associated with forming it from its most basic components. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1. Standard Enthalpy Of Formation Difference.
From www.linstitute.net
IB DP Chemistry SL复习笔记5.1.2 Standard Enthalpy Change翰林国际教育 Standard Enthalpy Of Formation Difference The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, take the reaction. Standard Enthalpy Of Formation Difference.
From pediaa.com
What is the Difference Between Enthalpy of Formation and Enthalpy of Standard Enthalpy Of Formation Difference The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its. Standard Enthalpy Of Formation Difference.
From studylib.net
Standard Enthalpy Formation Standard Enthalpy Of Formation Difference The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created. Standard Enthalpy Of Formation Difference.
From radenbayu33as.blogspot.com
Enthalpy Of Formation Of C3H8 Enthalpy Changes during Formation of Standard Enthalpy Of Formation Difference The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements.. Standard Enthalpy Of Formation Difference.
From www.slideserve.com
PPT Standard Enthalpy Changes = D H o PowerPoint Presentation, free Standard Enthalpy Of Formation Difference For example, take the reaction forming hexane (c. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. The standard enthalpy of formation, also known as. Standard Enthalpy Of Formation Difference.
From www.linstitute.net
CIE A Level Chemistry复习笔记5.1.7 Constructing Energy Cycles using Standard Enthalpy Of Formation Difference The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. Expressed differently, a molecule’s enthalpy of formation is the heat associated with forming it from its most basic components. For example, take the reaction forming hexane (c. The standard. Standard Enthalpy Of Formation Difference.
From www.youtube.com
6.14 Calculate the standard enthalpy of formation of CH3OH(l) from the Standard Enthalpy Of Formation Difference For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. Expressed differently, a molecule’s enthalpy of formation is the heat associated with forming it from its most basic components. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, take. Standard Enthalpy Of Formation Difference.
From www.slideserve.com
PPT Standard Enthalpies of Formation PowerPoint Presentation, free Standard Enthalpy Of Formation Difference 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. For example, take the reaction forming hexane (c. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. The standard enthalpy of formation, also known as the heat of formation, is the. Standard Enthalpy Of Formation Difference.
From schoolworkhelper.net
Standard Enthalpies of Formation, ΔH°f SchoolWorkHelper Standard Enthalpy Of Formation Difference The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. For example, although oxygen can exist. Standard Enthalpy Of Formation Difference.
From www.chegg.com
Solved The standard enthalpy of formation (ΔHf∘) is the Standard Enthalpy Of Formation Difference For example, take the reaction forming hexane (c. Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when. Expressed differently, a molecule’s enthalpy of formation is the heat associated with forming it from its most basic components. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation. Standard Enthalpy Of Formation Difference.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Difference Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. Expressed differently, a molecule’s enthalpy of formation is the heat associated with forming it from its most basic components. The standard enthalpy. Standard Enthalpy Of Formation Difference.
From www.writework.com
A comparison between the Enthalpy of formation of MgO acquired via a Standard Enthalpy Of Formation Difference For example, take the reaction forming hexane (c. Expressed differently, a molecule’s enthalpy of formation is the heat associated with forming it from its most basic components. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation, also known as the heat. Standard Enthalpy Of Formation Difference.
From priaxon.com
What Is Standard Enthalpy Of Formation Of Nh3 Gas Templates Printable Standard Enthalpy Of Formation Difference The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms. Standard Enthalpy Of Formation Difference.
From pdfprof.com
enthalpies standard de formation et entropie standard Standard Enthalpy Of Formation Difference Expressed differently, a molecule’s enthalpy of formation is the heat associated with forming it from its most basic components. Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed from its elements. For example, although. Standard Enthalpy Of Formation Difference.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation Difference The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation is the enthalpy change when 1 mol of. Standard Enthalpy Of Formation Difference.
From www.numerade.com
SOLVED What is the difference between the standard enthalpy of Standard Enthalpy Of Formation Difference The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Yes, the standard enthalpy of reaction. Standard Enthalpy Of Formation Difference.
From www.slideserve.com
PPT Heat of Formation PowerPoint Presentation, free download ID3890043 Standard Enthalpy Of Formation Difference For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. The standard enthalpy of formation of any element in its standard state. Standard Enthalpy Of Formation Difference.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Difference For example, take the reaction forming hexane (c. Expressed differently, a molecule’s enthalpy of formation is the heat associated with forming it from its most basic components. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. The standard enthalpy of formation is the enthalpy change when 1 mol of a pure substance is formed. Standard Enthalpy Of Formation Difference.
From www.nagwa.com
Question Video Calculating the Standard Enthalpy of Formation for Standard Enthalpy Of Formation Difference 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. The standard enthalpy of formation. Standard Enthalpy Of Formation Difference.
From www.researchgate.net
Differences in the enthalpy of formation ∆E [kcal/mol] of AB194 isomers Standard Enthalpy Of Formation Difference The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. For example, take the reaction forming hexane (c. The standard enthalpy of. Standard Enthalpy Of Formation Difference.
From www.researchgate.net
Enthalpies of formation for stable and radical species used in work Standard Enthalpy Of Formation Difference Expressed differently, a molecule’s enthalpy of formation is the heat associated with forming it from its most basic components. Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change that occurs in a system when. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms. Standard Enthalpy Of Formation Difference.
From www.congress-intercultural.eu
Different Types Of Heat (Enthalpy) Of Reaction Read, 42 OFF Standard Enthalpy Of Formation Difference The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is a measure of the energy released or consumed when one mole of a substance is created under standard. For example, take the reaction forming hexane (c. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or. Standard Enthalpy Of Formation Difference.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Difference For example, take the reaction forming hexane (c. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is the enthalpy change when 1 mol of. Standard Enthalpy Of Formation Difference.
From www.studocu.com
Standard Enthalpy of Formation Table Standard Enthalpy of Formation Standard Enthalpy Of Formation Difference The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation, also known as the heat of formation, is the enthalpy change when 1 mole of a pure substance forms from its constituent elements at standard temperature and pressure. Yes, the standard enthalpy of reaction ($\delta_\mathrm{r}h^\circ$) is the enthalpy change. Standard Enthalpy Of Formation Difference.
From www.youtube.com
Standard Enthalpy of Formation and Formation Reactions OpenStax Standard Enthalpy Of Formation Difference 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. The standard enthalpy of formation of any element in its standard state is zero by definition. For example, although oxygen can exist as ozone (o 3 ), atomic oxygen (o), and. The standard enthalpy of formation, also. Standard Enthalpy Of Formation Difference.