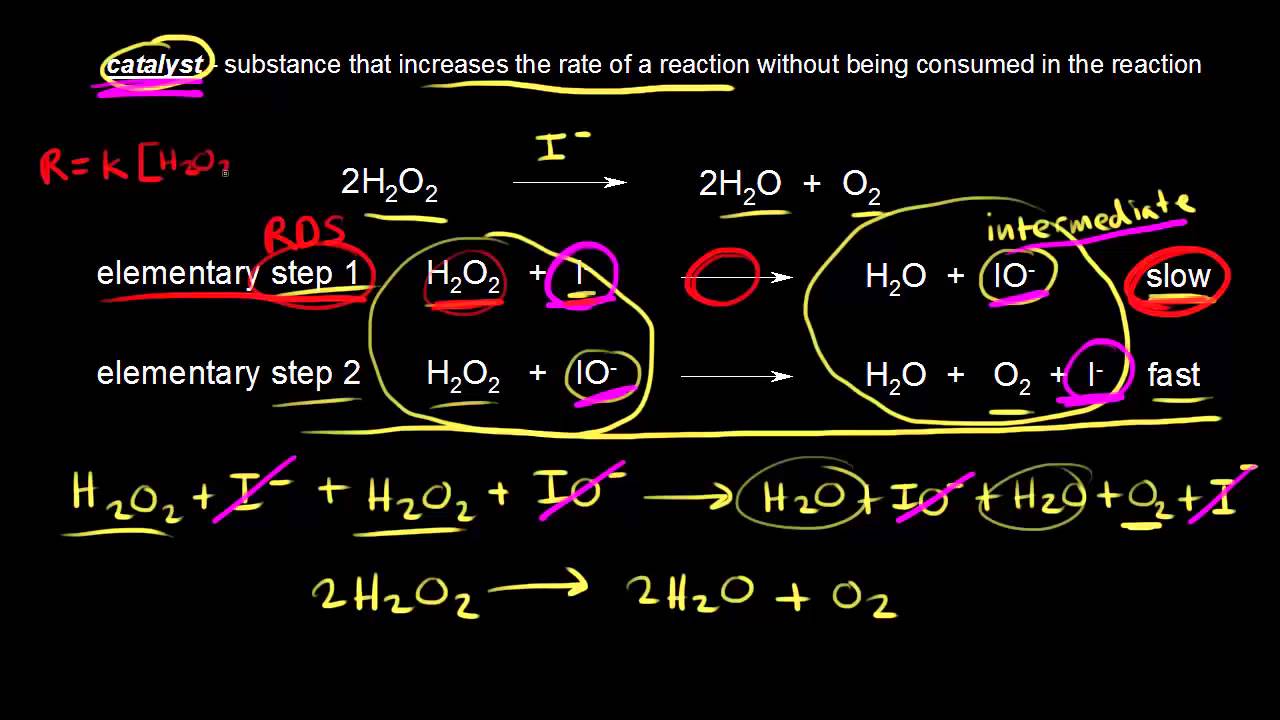
How Does Catalyst Chemical Reaction Work . A catalyst provides an alternative. what are chemical absorptions and how do they promote chemical reactions? Why metal clusters will be excellent potential catalysts? How transition metals are chosen as catalysts? a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. What types of chemisorption lead to the poisoning of a catalyst? The catalyst is not used up or chemically changed. A catalyst is a substance that speeds up a chemical reaction. what are catalysts, and how do they work in terms altering the parameters of a reaction? catalysts participate in a chemical reaction and increase its rate. What are syngases and how are they prepared? catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. They do not appear in the reaction’s net equation and are not consumed during the.
from www.youtube.com
a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. Why metal clusters will be excellent potential catalysts? The catalyst is not used up or chemically changed. What types of chemisorption lead to the poisoning of a catalyst? catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. what are chemical absorptions and how do they promote chemical reactions? A catalyst provides an alternative. How transition metals are chosen as catalysts? A catalyst is a substance that speeds up a chemical reaction. catalysts participate in a chemical reaction and increase its rate.
Catalysts AP Chemistry Khan Academy YouTube
How Does Catalyst Chemical Reaction Work They do not appear in the reaction’s net equation and are not consumed during the. How transition metals are chosen as catalysts? A catalyst provides an alternative. catalysts participate in a chemical reaction and increase its rate. a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. Why metal clusters will be excellent potential catalysts? A catalyst is a substance that speeds up a chemical reaction. What types of chemisorption lead to the poisoning of a catalyst? what are chemical absorptions and how do they promote chemical reactions? catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. They do not appear in the reaction’s net equation and are not consumed during the. The catalyst is not used up or chemically changed. what are catalysts, and how do they work in terms altering the parameters of a reaction? What are syngases and how are they prepared?
From www.expii.com
Catalysts (Enzymes) — Overview & Examples Expii How Does Catalyst Chemical Reaction Work The catalyst is not used up or chemically changed. A catalyst is a substance that speeds up a chemical reaction. what are catalysts, and how do they work in terms altering the parameters of a reaction? Why metal clusters will be excellent potential catalysts? How transition metals are chosen as catalysts? catalysts participate in a chemical reaction and. How Does Catalyst Chemical Reaction Work.
From www.researchgate.net
1 Schematic illustration of a catalytic process showing "A" and "B How Does Catalyst Chemical Reaction Work a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. Why metal clusters will be excellent potential catalysts? catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. what are chemical absorptions and how do they promote chemical reactions? How. How Does Catalyst Chemical Reaction Work.
From www.slideserve.com
PPT IGCSE Chemistry PowerPoint Presentation, free download ID5408111 How Does Catalyst Chemical Reaction Work catalysts participate in a chemical reaction and increase its rate. What are syngases and how are they prepared? A catalyst is a substance that speeds up a chemical reaction. They do not appear in the reaction’s net equation and are not consumed during the. a catalyst binds to a reactant and it increases the number of collision between. How Does Catalyst Chemical Reaction Work.
From www.dreamstime.com
Catalyst Surface with Catalytic Reaction Stock Vector Illustration of How Does Catalyst Chemical Reaction Work catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. The catalyst is not used up or chemically changed. what are catalysts, and how do they work in terms altering. How Does Catalyst Chemical Reaction Work.
From www.slideserve.com
PPT Chapter 11 Chemical Reactions PowerPoint Presentation, free How Does Catalyst Chemical Reaction Work what are chemical absorptions and how do they promote chemical reactions? What are syngases and how are they prepared? what are catalysts, and how do they work in terms altering the parameters of a reaction? catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. catalysts participate in a chemical. How Does Catalyst Chemical Reaction Work.
From www.dreamstime.com
Enzyme As Catalyst in Chemical Reactions Stock Vector Illustration of How Does Catalyst Chemical Reaction Work A catalyst is a substance that speeds up a chemical reaction. What types of chemisorption lead to the poisoning of a catalyst? The catalyst is not used up or chemically changed. They do not appear in the reaction’s net equation and are not consumed during the. What are syngases and how are they prepared? what are catalysts, and how. How Does Catalyst Chemical Reaction Work.
From www.youtube.com
How does a CATALYST work ? YouTube How Does Catalyst Chemical Reaction Work A catalyst provides an alternative. Why metal clusters will be excellent potential catalysts? What types of chemisorption lead to the poisoning of a catalyst? what are catalysts, and how do they work in terms altering the parameters of a reaction? They do not appear in the reaction’s net equation and are not consumed during the. catalysts participate in. How Does Catalyst Chemical Reaction Work.
From www.youtube.com
Catalytic Converter Working Principle 2 way and 3 way, Function of How Does Catalyst Chemical Reaction Work The catalyst is not used up or chemically changed. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. A catalyst provides an alternative. What types of chemisorption lead to the. How Does Catalyst Chemical Reaction Work.
From www.slideserve.com
PPT Mechanisms, Catalysts Intermediates and k PowerPoint Presentation How Does Catalyst Chemical Reaction Work a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst provides an alternative. Why metal clusters will be excellent potential catalysts? A catalyst is a substance that speeds up. How Does Catalyst Chemical Reaction Work.
From fyoulxtwt.blob.core.windows.net
How Do Catalysts Work Gcse at Sharon Santucci blog How Does Catalyst Chemical Reaction Work The catalyst is not used up or chemically changed. A catalyst is a substance that speeds up a chemical reaction. They do not appear in the reaction’s net equation and are not consumed during the. catalysts participate in a chemical reaction and increase its rate. a catalyst binds to a reactant and it increases the number of collision. How Does Catalyst Chemical Reaction Work.
From www.savemyexams.com
Types of Catalyst CIE A Level Chemistry Revision Notes 2025 How Does Catalyst Chemical Reaction Work How transition metals are chosen as catalysts? what are catalysts, and how do they work in terms altering the parameters of a reaction? What are syngases and how are they prepared? Why metal clusters will be excellent potential catalysts? catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst is. How Does Catalyst Chemical Reaction Work.
From www.youtube.com
Catalysts AP Chemistry Khan Academy YouTube How Does Catalyst Chemical Reaction Work A catalyst is a substance that speeds up a chemical reaction. The catalyst is not used up or chemically changed. what are chemical absorptions and how do they promote chemical reactions? What are syngases and how are they prepared? a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the. How Does Catalyst Chemical Reaction Work.
From www.pinterest.com
Catalyst speeds up a chemical reaction by lowering the activation How Does Catalyst Chemical Reaction Work Why metal clusters will be excellent potential catalysts? What types of chemisorption lead to the poisoning of a catalyst? How transition metals are chosen as catalysts? What are syngases and how are they prepared? They do not appear in the reaction’s net equation and are not consumed during the. catalysts participate in a chemical reaction and increase its rate.. How Does Catalyst Chemical Reaction Work.
From www.slideserve.com
PPT ENZYME BIOLOGICAL CATALYST PowerPoint Presentation, free How Does Catalyst Chemical Reaction Work catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. A catalyst provides an alternative. They do not appear in the reaction’s net equation and are not consumed during the. what are catalysts, and how do they work in terms altering the parameters of a reaction? How transition metals are chosen as. How Does Catalyst Chemical Reaction Work.
From www.thoughtco.com
Catalysis Definition in Chemistry How Does Catalyst Chemical Reaction Work A catalyst provides an alternative. How transition metals are chosen as catalysts? The catalyst is not used up or chemically changed. Why metal clusters will be excellent potential catalysts? what are catalysts, and how do they work in terms altering the parameters of a reaction? catalysts participate in a chemical reaction and increase its rate. They do not. How Does Catalyst Chemical Reaction Work.
From www.researchgate.net
Reaction coordinate diagram showing the working principle of a catalyst How Does Catalyst Chemical Reaction Work A catalyst provides an alternative. How transition metals are chosen as catalysts? A catalyst is a substance that speeds up a chemical reaction. The catalyst is not used up or chemically changed. what are catalysts, and how do they work in terms altering the parameters of a reaction? Why metal clusters will be excellent potential catalysts? catalyst, in. How Does Catalyst Chemical Reaction Work.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited How Does Catalyst Chemical Reaction Work a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. The catalyst is not used up or chemically changed. what are chemical absorptions and how do they promote chemical reactions? Why metal clusters will be excellent potential catalysts? A catalyst is a substance that speeds up a. How Does Catalyst Chemical Reaction Work.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub How Does Catalyst Chemical Reaction Work a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. A catalyst is a substance that speeds up a chemical reaction. The catalyst is not used up or chemically changed. A catalyst provides an alternative. What are syngases and how are they prepared? catalyst, in chemistry, any. How Does Catalyst Chemical Reaction Work.
From www.labunlimited.com
Solid Phase Catalysis in Continuous Flow Chemistry Lab Unlimited How Does Catalyst Chemical Reaction Work A catalyst provides an alternative. A catalyst is a substance that speeds up a chemical reaction. a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. catalysts participate in a chemical reaction and increase its rate. The catalyst is not used up or chemically changed. Why metal. How Does Catalyst Chemical Reaction Work.
From derekcarrsavvy-chemist.blogspot.com
savvychemist GCSE OCR Gateway Chemistry C5.2 fi Catalysis and catalysts How Does Catalyst Chemical Reaction Work a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. What types of chemisorption lead to the poisoning of a catalyst? What are syngases and how are they prepared? what are catalysts, and how do they work in terms altering the parameters of a reaction? How transition. How Does Catalyst Chemical Reaction Work.
From www.slideshare.net
Biology 2.4 How Does Catalyst Chemical Reaction Work They do not appear in the reaction’s net equation and are not consumed during the. catalysts participate in a chemical reaction and increase its rate. What types of chemisorption lead to the poisoning of a catalyst? Why metal clusters will be excellent potential catalysts? what are catalysts, and how do they work in terms altering the parameters of. How Does Catalyst Chemical Reaction Work.
From www.mometrix.com
What is a Catalyst? Chemistry Review (Video) How Does Catalyst Chemical Reaction Work How transition metals are chosen as catalysts? a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. A catalyst is a substance that speeds up a chemical reaction. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. What are syngases. How Does Catalyst Chemical Reaction Work.
From www.catalystseurope.org
What are catalysts? How Does Catalyst Chemical Reaction Work Why metal clusters will be excellent potential catalysts? what are catalysts, and how do they work in terms altering the parameters of a reaction? The catalyst is not used up or chemically changed. catalysts participate in a chemical reaction and increase its rate. How transition metals are chosen as catalysts? What are syngases and how are they prepared?. How Does Catalyst Chemical Reaction Work.
From newenergyandfuel.com
Seeing a Catalytic Chemical Reaction in Real Time How Does Catalyst Chemical Reaction Work What are syngases and how are they prepared? A catalyst provides an alternative. How transition metals are chosen as catalysts? what are catalysts, and how do they work in terms altering the parameters of a reaction? what are chemical absorptions and how do they promote chemical reactions? Why metal clusters will be excellent potential catalysts? a catalyst. How Does Catalyst Chemical Reaction Work.
From fyoulxtwt.blob.core.windows.net
How Do Catalysts Work Gcse at Sharon Santucci blog How Does Catalyst Chemical Reaction Work What are syngases and how are they prepared? A catalyst is a substance that speeds up a chemical reaction. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. What types of chemisorption lead to the poisoning of a catalyst? A catalyst provides an alternative. what are catalysts, and how do they. How Does Catalyst Chemical Reaction Work.
From www.chemengonline.com
Catalysis Fundamentals Chemical Engineering Page 1 How Does Catalyst Chemical Reaction Work what are catalysts, and how do they work in terms altering the parameters of a reaction? Why metal clusters will be excellent potential catalysts? A catalyst provides an alternative. What types of chemisorption lead to the poisoning of a catalyst? What are syngases and how are they prepared? How transition metals are chosen as catalysts? catalysts participate in. How Does Catalyst Chemical Reaction Work.
From www.researchgate.net
Catalytic processes on a solid catalyst. Download Scientific Diagram How Does Catalyst Chemical Reaction Work what are chemical absorptions and how do they promote chemical reactions? They do not appear in the reaction’s net equation and are not consumed during the. Why metal clusters will be excellent potential catalysts? what are catalysts, and how do they work in terms altering the parameters of a reaction? A catalyst provides an alternative. catalyst, in. How Does Catalyst Chemical Reaction Work.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis How Does Catalyst Chemical Reaction Work what are catalysts, and how do they work in terms altering the parameters of a reaction? Why metal clusters will be excellent potential catalysts? what are chemical absorptions and how do they promote chemical reactions? The catalyst is not used up or chemically changed. a catalyst binds to a reactant and it increases the number of collision. How Does Catalyst Chemical Reaction Work.
From fyoerprzq.blob.core.windows.net
What Are Catalysts In Chemical Reactions at Brian Edwards blog How Does Catalyst Chemical Reaction Work catalysts participate in a chemical reaction and increase its rate. what are chemical absorptions and how do they promote chemical reactions? Why metal clusters will be excellent potential catalysts? a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. How transition metals are chosen as catalysts?. How Does Catalyst Chemical Reaction Work.
From www.youtube.com
What Are Catalysts? Reactions Chemistry FuseSchool YouTube How Does Catalyst Chemical Reaction Work a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. A catalyst provides an alternative. what are catalysts, and how do they work in terms altering the parameters of a reaction? What are syngases and how are they prepared? what are chemical absorptions and how do. How Does Catalyst Chemical Reaction Work.
From www.slideserve.com
PPT Fast and Slow Chemistry PowerPoint Presentation, free download How Does Catalyst Chemical Reaction Work catalysts participate in a chemical reaction and increase its rate. Why metal clusters will be excellent potential catalysts? a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. A catalyst is a substance that speeds up a chemical reaction. They do not appear in the reaction’s net. How Does Catalyst Chemical Reaction Work.
From www.youtube.com
A Catalyst and the Rate of Reaction YouTube How Does Catalyst Chemical Reaction Work A catalyst provides an alternative. The catalyst is not used up or chemically changed. what are chemical absorptions and how do they promote chemical reactions? What types of chemisorption lead to the poisoning of a catalyst? Why metal clusters will be excellent potential catalysts? What are syngases and how are they prepared? catalysts participate in a chemical reaction. How Does Catalyst Chemical Reaction Work.
From www.britannica.com
Catalyst Examples, Definition, & Facts Britannica How Does Catalyst Chemical Reaction Work What types of chemisorption lead to the poisoning of a catalyst? A catalyst provides an alternative. What are syngases and how are they prepared? They do not appear in the reaction’s net equation and are not consumed during the. a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction. How Does Catalyst Chemical Reaction Work.
From www.pinterest.com
Homogeneous Catalyst Easy Science Ap chemistry, Chemical equation How Does Catalyst Chemical Reaction Work Why metal clusters will be excellent potential catalysts? What types of chemisorption lead to the poisoning of a catalyst? a catalyst binds to a reactant and it increases the number of collision between the reactant molecules, making the reaction more. A catalyst is a substance that speeds up a chemical reaction. The catalyst is not used up or chemically. How Does Catalyst Chemical Reaction Work.
From www.youtube.com
Identifying catalysts in a reaction YouTube How Does Catalyst Chemical Reaction Work what are chemical absorptions and how do they promote chemical reactions? The catalyst is not used up or chemically changed. What are syngases and how are they prepared? Why metal clusters will be excellent potential catalysts? A catalyst is a substance that speeds up a chemical reaction. a catalyst binds to a reactant and it increases the number. How Does Catalyst Chemical Reaction Work.