Lab 2 Ph And Buffers . study with quizlet and memorize flashcards containing terms like ph, how to use ph indicator strips, saliva ph and more. Ph affects the structure and activity of biological macromolecules; A weak acid and it’s conjugate base in equilibrium: When the ph = 7, the solution is. to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume change) at ph 7, compared to adding it to 1.0 liter of a 1m acetate buffer at ph 4.76. Biology archive > unit 2. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and. buffers are solutions that resist changes in ph when small amounts of acid or base are added. For example, the catalytic activity of enzymes is. • basic solution is when.
from www.studocu.com
For example, the catalytic activity of enzymes is. • basic solution is when. buffers are solutions that resist changes in ph when small amounts of acid or base are added. Biology archive > unit 2. study with quizlet and memorize flashcards containing terms like ph, how to use ph indicator strips, saliva ph and more. Ph affects the structure and activity of biological macromolecules; When the ph = 7, the solution is. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and. to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume change) at ph 7, compared to adding it to 1.0 liter of a 1m acetate buffer at ph 4.76. A weak acid and it’s conjugate base in equilibrium:
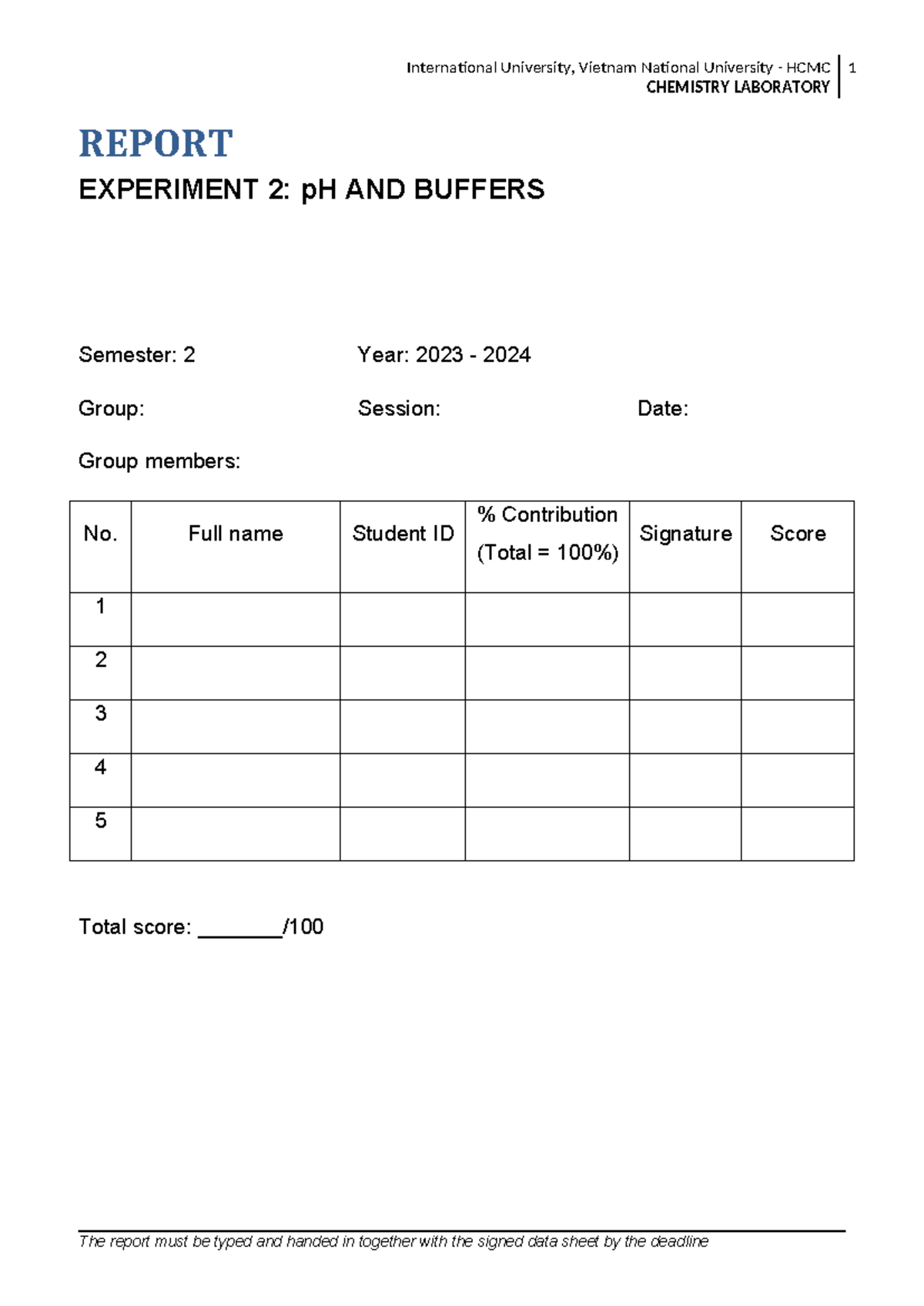
Chem Lab Report 2 Guideline CHEMISTRY LABORATORY REPORT EXPERIMENT
Lab 2 Ph And Buffers When the ph = 7, the solution is. For example, the catalytic activity of enzymes is. When the ph = 7, the solution is. study with quizlet and memorize flashcards containing terms like ph, how to use ph indicator strips, saliva ph and more. • basic solution is when. A weak acid and it’s conjugate base in equilibrium: vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and. buffers are solutions that resist changes in ph when small amounts of acid or base are added. to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume change) at ph 7, compared to adding it to 1.0 liter of a 1m acetate buffer at ph 4.76. Biology archive > unit 2. Ph affects the structure and activity of biological macromolecules;
From www.studocu.com
Report 2 p H AND Buffers lab 2 CHEMISTRY LABORATORY REPORT Lab 2 Ph And Buffers Biology archive > unit 2. study with quizlet and memorize flashcards containing terms like ph, how to use ph indicator strips, saliva ph and more. A weak acid and it’s conjugate base in equilibrium: For example, the catalytic activity of enzymes is. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic. Lab 2 Ph And Buffers.
From www.youtube.com
EXPERIMENT Preparation of buffer solution and measurement of pH Lab 2 Ph And Buffers vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and. For example, the catalytic activity of enzymes is. Biology archive > unit 2. Ph affects the structure and activity of biological macromolecules; study with quizlet and memorize flashcards containing terms like ph, how to use ph. Lab 2 Ph And Buffers.
From www.chegg.com
Solved REPORT SHEET LAB Acids, Bases, pH, and Buffers 19 PH Lab 2 Ph And Buffers For example, the catalytic activity of enzymes is. buffers are solutions that resist changes in ph when small amounts of acid or base are added. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and. to understand how well a buffer protects against changes in. Lab 2 Ph And Buffers.
From www.studocu.com
Lab 05 p H and Buffers Lab report. Lab pH and Buffers Lab 2 Ph And Buffers When the ph = 7, the solution is. to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume change) at ph 7, compared to adding it to 1.0 liter of a 1m acetate buffer at ph 4.76. Biology archive > unit 2.. Lab 2 Ph And Buffers.
From www.chegg.com
Solved Acids, Bases, Buffers and p Postlaboratory Lab 2 Ph And Buffers • basic solution is when. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and. Ph affects the structure and activity of biological macromolecules; study with quizlet and memorize flashcards containing terms like ph, how to use ph indicator strips, saliva ph and more. Biology. Lab 2 Ph And Buffers.
From www.chegg.com
Solved The lab is called "pH Buffers and Their Lab 2 Ph And Buffers buffers are solutions that resist changes in ph when small amounts of acid or base are added. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and. A weak acid and it’s conjugate base in equilibrium: • basic solution is when. When the ph =. Lab 2 Ph And Buffers.
From www.studocu.com
Chem Lab Report 2 Guideline CHEMISTRY LABORATORY REPORT EXPERIMENT Lab 2 Ph And Buffers • basic solution is when. to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume change) at ph 7, compared to adding it to 1.0 liter of a 1m acetate buffer at ph 4.76. vertebrate organisms maintain the ph of. Lab 2 Ph And Buffers.
From www.scribd.com
Experiment 2 PH and Buffers Group Section Date PDF Ph Lab 2 Ph And Buffers vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and. When the ph = 7, the solution is. Ph affects the structure and activity of biological macromolecules; buffers are solutions that resist changes in ph when small amounts of acid or base are added. For example,. Lab 2 Ph And Buffers.
From www.studocu.com
Oxidation and Reduction Post Lab POST LAB 2 pH and buffers Notes on Lab 2 Ph And Buffers study with quizlet and memorize flashcards containing terms like ph, how to use ph indicator strips, saliva ph and more. buffers are solutions that resist changes in ph when small amounts of acid or base are added. to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to. Lab 2 Ph And Buffers.
From www.chegg.com
Solved REPORT SHEET LAB Acids, Bases, pH, and Buffers 19 A. Lab 2 Ph And Buffers buffers are solutions that resist changes in ph when small amounts of acid or base are added. study with quizlet and memorize flashcards containing terms like ph, how to use ph indicator strips, saliva ph and more. to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to. Lab 2 Ph And Buffers.
From www.chegg.com
Acids, Bases, Buffers and pH Postlaboratory Lab 2 Ph And Buffers When the ph = 7, the solution is. • basic solution is when. study with quizlet and memorize flashcards containing terms like ph, how to use ph indicator strips, saliva ph and more. A weak acid and it’s conjugate base in equilibrium: Ph affects the structure and activity of biological macromolecules; vertebrate organisms maintain the ph of. Lab 2 Ph And Buffers.
From quizlet.com
Lab 2 pH and Buffers Diagram Quizlet Lab 2 Ph And Buffers vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and. buffers are solutions that resist changes in ph when small amounts of acid or base are added. For example, the catalytic activity of enzymes is. Biology archive > unit 2. study with quizlet and memorize. Lab 2 Ph And Buffers.
From www.chegg.com
LAB 2 pH AND BUFFERS Procedure 5.1 Measuring the pH Lab 2 Ph And Buffers buffers are solutions that resist changes in ph when small amounts of acid or base are added. When the ph = 7, the solution is. study with quizlet and memorize flashcards containing terms like ph, how to use ph indicator strips, saliva ph and more. • basic solution is when. A weak acid and it’s conjugate base. Lab 2 Ph And Buffers.
From www.chegg.com
Solved Lab 3 pH and Buffers Discussion A. The pH scale and Lab 2 Ph And Buffers Ph affects the structure and activity of biological macromolecules; vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and. buffers are solutions that resist changes in ph when small amounts of acid or base are added. • basic solution is when. Biology archive > unit. Lab 2 Ph And Buffers.
From www.studypool.com
SOLUTION Lab report ph and buffers Studypool Lab 2 Ph And Buffers to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume change) at ph 7, compared to adding it to 1.0 liter of a 1m acetate buffer at ph 4.76. For example, the catalytic activity of enzymes is. • basic solution is. Lab 2 Ph And Buffers.
From www.studocu.com
Lab 2 Chem lab Lab 2 Chem lab CHEMISTRY LABORATORY REPORT Lab 2 Ph And Buffers • basic solution is when. A weak acid and it’s conjugate base in equilibrium: Ph affects the structure and activity of biological macromolecules; to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume change) at ph 7, compared to adding it. Lab 2 Ph And Buffers.
From www.studocu.com
Instruction Expt. 2p H and Buffers EXPERIMENT 2 ‐ pH AND BUFFERS 1 Lab 2 Ph And Buffers buffers are solutions that resist changes in ph when small amounts of acid or base are added. Biology archive > unit 2. For example, the catalytic activity of enzymes is. to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume change). Lab 2 Ph And Buffers.
From www.studypool.com
SOLUTION Ph and buffer system Lab Report Studypool Lab 2 Ph And Buffers to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume change) at ph 7, compared to adding it to 1.0 liter of a 1m acetate buffer at ph 4.76. • basic solution is when. buffers are solutions that resist changes. Lab 2 Ph And Buffers.
From www.studocu.com
Lab 2 ph and buffers (with prelab quiz 2) BI 201 Studocu Lab 2 Ph And Buffers When the ph = 7, the solution is. • basic solution is when. Biology archive > unit 2. buffers are solutions that resist changes in ph when small amounts of acid or base are added. For example, the catalytic activity of enzymes is. study with quizlet and memorize flashcards containing terms like ph, how to use ph. Lab 2 Ph And Buffers.
From www.studocu.com
LAB 2 p H and Buffers Chemistry LAB 2 pH AND BUFFERS Name Mayelin Lab 2 Ph And Buffers Biology archive > unit 2. For example, the catalytic activity of enzymes is. Ph affects the structure and activity of biological macromolecules; vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and. buffers are solutions that resist changes in ph when small amounts of acid or. Lab 2 Ph And Buffers.
From www.chegg.com
Solved Lab Report pH and Buffers Part II, need help with Lab 2 Ph And Buffers study with quizlet and memorize flashcards containing terms like ph, how to use ph indicator strips, saliva ph and more. For example, the catalytic activity of enzymes is. buffers are solutions that resist changes in ph when small amounts of acid or base are added. Ph affects the structure and activity of biological macromolecules; to understand how. Lab 2 Ph And Buffers.
From www.transtutors.com
(Solved) REPORT SHEET LAB Acids, Bases, pH, and Buffers 19 A Lab 2 Ph And Buffers Ph affects the structure and activity of biological macromolecules; For example, the catalytic activity of enzymes is. study with quizlet and memorize flashcards containing terms like ph, how to use ph indicator strips, saliva ph and more. to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0. Lab 2 Ph And Buffers.
From www.scribd.com
Experiment 2 PH and Buffers 1. Objectives PDF Acid Hydroxide Lab 2 Ph And Buffers • basic solution is when. to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume change) at ph 7, compared to adding it to 1.0 liter of a 1m acetate buffer at ph 4.76. study with quizlet and memorize flashcards. Lab 2 Ph And Buffers.
From www.slideserve.com
PPT Lab Activity 2 Active Acidity, pH, and Buffer PowerPoint Lab 2 Ph And Buffers Biology archive > unit 2. to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume change) at ph 7, compared to adding it to 1.0 liter of a 1m acetate buffer at ph 4.76. For example, the catalytic activity of enzymes is.. Lab 2 Ph And Buffers.
From www.studocu.com
Report 2 CHEMISTRY LABORATORY REPORT EXPERIMENT 2 pH AND BUFFERS Lab 2 Ph And Buffers For example, the catalytic activity of enzymes is. to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume change) at ph 7, compared to adding it to 1.0 liter of a 1m acetate buffer at ph 4.76. Biology archive > unit 2.. Lab 2 Ph And Buffers.
From www.numerade.com
SOLVED REPORT SHEET LAB Acids, Bases, pH, and Buffers Reference Lab 2 Ph And Buffers When the ph = 7, the solution is. study with quizlet and memorize flashcards containing terms like ph, how to use ph indicator strips, saliva ph and more. buffers are solutions that resist changes in ph when small amounts of acid or base are added. to understand how well a buffer protects against changes in ph, consider. Lab 2 Ph And Buffers.
From www.scribd.com
Experiment 1 PH and Buffers Pre Lab PDF Buffer Solution Acid Lab 2 Ph And Buffers • basic solution is when. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and. buffers are solutions that resist changes in ph when small amounts of acid or base are added. Ph affects the structure and activity of biological macromolecules; When the ph =. Lab 2 Ph And Buffers.
From www.scribd.com
pH and Buffers Acid Buffer Solution Lab 2 Ph And Buffers Ph affects the structure and activity of biological macromolecules; A weak acid and it’s conjugate base in equilibrium: Biology archive > unit 2. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and. For example, the catalytic activity of enzymes is. • basic solution is when.. Lab 2 Ph And Buffers.
From fyojkjzvi.blob.core.windows.net
Hydrolysis Of Salts And Ph Of Buffer Solutions Lab Report at Martin Lab 2 Ph And Buffers • basic solution is when. buffers are solutions that resist changes in ph when small amounts of acid or base are added. Ph affects the structure and activity of biological macromolecules; study with quizlet and memorize flashcards containing terms like ph, how to use ph indicator strips, saliva ph and more. vertebrate organisms maintain the ph. Lab 2 Ph And Buffers.
From www.studypool.com
SOLUTION Ph and buffer system Lab Report Studypool Lab 2 Ph And Buffers Biology archive > unit 2. Ph affects the structure and activity of biological macromolecules; study with quizlet and memorize flashcards containing terms like ph, how to use ph indicator strips, saliva ph and more. When the ph = 7, the solution is. buffers are solutions that resist changes in ph when small amounts of acid or base are. Lab 2 Ph And Buffers.
From www.studocu.com
Lab 2 lab 2 Lab 2 Page 1 of 7 Laboratory 2 pH and Buffer Lab 2 Ph And Buffers vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and. study with quizlet and memorize flashcards containing terms like ph, how to use ph indicator strips, saliva ph and more. Ph affects the structure and activity of biological macromolecules; A weak acid and it’s conjugate base. Lab 2 Ph And Buffers.
From www.chegg.com
Unit 4, Lab 1 Acids, Bases, and pH Buffers Date Lab 2 Ph And Buffers • basic solution is when. For example, the catalytic activity of enzymes is. to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume change) at ph 7, compared to adding it to 1.0 liter of a 1m acetate buffer at ph. Lab 2 Ph And Buffers.
From www.scribd.com
Lab 2 PH and Buffers 2022 PDF Lab 2 Ph And Buffers A weak acid and it’s conjugate base in equilibrium: to understand how well a buffer protects against changes in ph, consider the effect of adding.01 moles of hcl to 1.0 liter of pure water (no volume change) at ph 7, compared to adding it to 1.0 liter of a 1m acetate buffer at ph 4.76. Ph affects the structure. Lab 2 Ph And Buffers.
From www.studocu.com
Lab 2 The scientific Method Buffer Lab 2 Buffers. Worth = (points Lab 2 Ph And Buffers buffers are solutions that resist changes in ph when small amounts of acid or base are added. • basic solution is when. study with quizlet and memorize flashcards containing terms like ph, how to use ph indicator strips, saliva ph and more. A weak acid and it’s conjugate base in equilibrium: Ph affects the structure and activity. Lab 2 Ph And Buffers.
From www.chegg.com
Solved pH and Buffer lab reportI need help with question Lab 2 Ph And Buffers Biology archive > unit 2. buffers are solutions that resist changes in ph when small amounts of acid or base are added. vertebrate organisms maintain the ph of blood using a buffer composed of a mixture of carbonic acid (h 2 co 3) and. A weak acid and it’s conjugate base in equilibrium: Ph affects the structure and. Lab 2 Ph And Buffers.