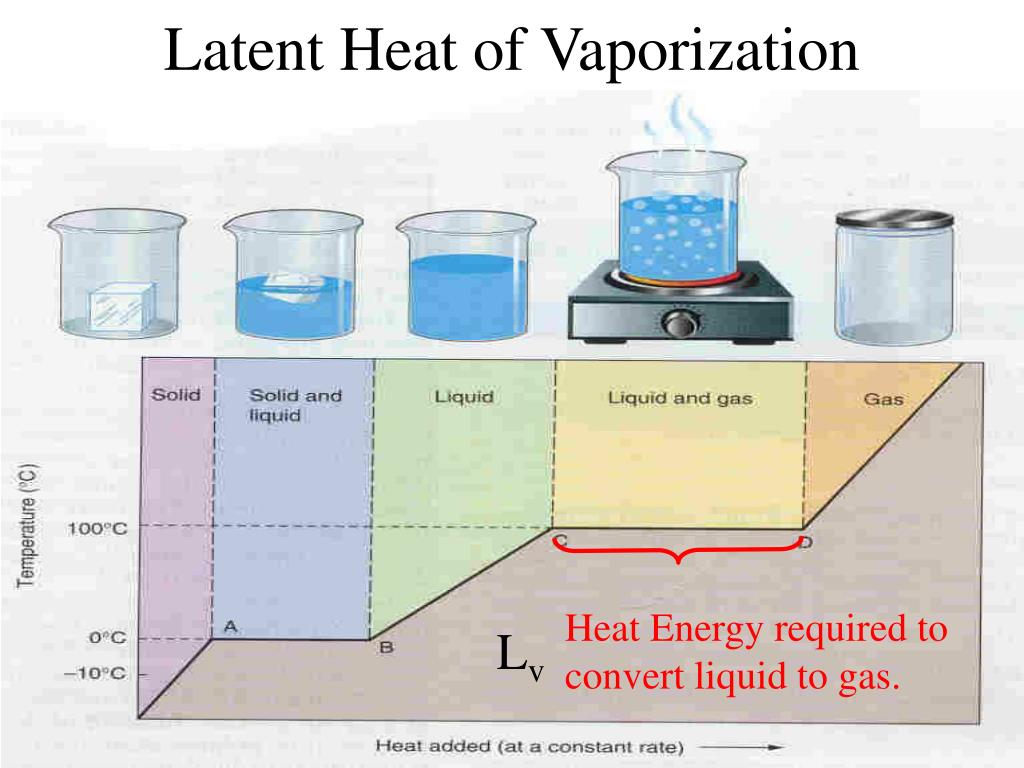
What S The Meaning Of Heat Of Vaporization . the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and. the heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid. the heat of vaporization is equal to the thermal energy required for vaporization divided by the mass of the substance that is vaporizing. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. the heat of vaporization is the amount of energy required to convert or evaporate a specific amount of a substance from a. the heat of vaporization is the quantity of heat that needs to be absorbed to vaporize a particular quantity of liquid at a constant.
from www.slideserve.com
the heat of vaporization is the quantity of heat that needs to be absorbed to vaporize a particular quantity of liquid at a constant. the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and. the heat of vaporization is the amount of energy required to convert or evaporate a specific amount of a substance from a. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. the heat of vaporization is equal to the thermal energy required for vaporization divided by the mass of the substance that is vaporizing. the heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid.
PPT Heat PowerPoint Presentation, free download ID5769198
What S The Meaning Of Heat Of Vaporization the heat of vaporization is the quantity of heat that needs to be absorbed to vaporize a particular quantity of liquid at a constant. the heat of vaporization is equal to the thermal energy required for vaporization divided by the mass of the substance that is vaporizing. the heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid. the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and. the heat of vaporization is the amount of energy required to convert or evaporate a specific amount of a substance from a. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. the heat of vaporization is the quantity of heat that needs to be absorbed to vaporize a particular quantity of liquid at a constant.
From engineerexcel.com
Heat of Vaporization Explained EngineerExcel What S The Meaning Of Heat Of Vaporization the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. the heat of vaporization is the amount of energy required to convert or evaporate a specific amount of a substance from a. the heat (or enthalpy) of vaporization is the quantity of heat that must be. What S The Meaning Of Heat Of Vaporization.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download What S The Meaning Of Heat Of Vaporization the heat of vaporization is the amount of energy required to convert or evaporate a specific amount of a substance from a. the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and. the molar heat of vaporization \(\left( \delta h_\text{vap}. What S The Meaning Of Heat Of Vaporization.
From www.slideserve.com
PPT Latent Heat PowerPoint Presentation ID5759776 What S The Meaning Of Heat Of Vaporization the heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid. the heat of vaporization is the amount of energy required to convert or evaporate a specific amount of a substance from a. the heat of vaporization is the enthalpy change when a unit mass of a. What S The Meaning Of Heat Of Vaporization.
From www.youtube.com
Heat of Vaporization from Vapor Pressure (Example) YouTube What S The Meaning Of Heat Of Vaporization the heat of vaporization is equal to the thermal energy required for vaporization divided by the mass of the substance that is vaporizing. the heat of vaporization is the amount of energy required to convert or evaporate a specific amount of a substance from a. the heat (or enthalpy) of vaporization is the quantity of heat that. What S The Meaning Of Heat Of Vaporization.
From www.tec-science.com
Specific latent heat of vaporization tecscience What S The Meaning Of Heat Of Vaporization the heat of vaporization is the amount of energy required to convert or evaporate a specific amount of a substance from a. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. the heat of vaporization is equal to the thermal energy required for vaporization divided. What S The Meaning Of Heat Of Vaporization.
From www.slideserve.com
PPT Change of State PowerPoint Presentation, free download ID1562618 What S The Meaning Of Heat Of Vaporization the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and. the heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\). What S The Meaning Of Heat Of Vaporization.
From www.slideserve.com
PPT Chapter 12 Liquids and Solids PowerPoint Presentation, free What S The Meaning Of Heat Of Vaporization the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and. the heat of vaporization is the amount of energy required to convert or evaporate a specific amount of a substance from a. the heat of vaporization is equal to the. What S The Meaning Of Heat Of Vaporization.
From www.geeksforgeeks.org
What is Vaporization? What S The Meaning Of Heat Of Vaporization the heat of vaporization is the amount of energy required to convert or evaporate a specific amount of a substance from a. the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and. the heat (or enthalpy) of vaporization is the. What S The Meaning Of Heat Of Vaporization.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation ID245553 What S The Meaning Of Heat Of Vaporization the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. the heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid. the heat of vaporization is the quantity of heat that needs to be absorbed to. What S The Meaning Of Heat Of Vaporization.
From www.slideserve.com
PPT States of Matter PowerPoint Presentation, free download ID5735591 What S The Meaning Of Heat Of Vaporization the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. the heat of vaporization is the quantity of heat that needs to be absorbed to vaporize a particular quantity of liquid at a constant. the heat of vaporization is equal to the thermal energy required for. What S The Meaning Of Heat Of Vaporization.
From www.slideserve.com
PPT Heat of Fusion and Vaporization PowerPoint Presentation, free What S The Meaning Of Heat Of Vaporization the heat of vaporization is equal to the thermal energy required for vaporization divided by the mass of the substance that is vaporizing. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. the heat of vaporization is the enthalpy change when a unit mass of. What S The Meaning Of Heat Of Vaporization.
From www.slideserve.com
PPT Biochemistry Unit PowerPoint Presentation, free download ID4550740 What S The Meaning Of Heat Of Vaporization the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and. the heat of vaporization is the quantity of heat that needs to be absorbed to vaporize a particular quantity of liquid at a constant. the heat (or enthalpy) of vaporization. What S The Meaning Of Heat Of Vaporization.
From www.slideserve.com
PPT 2 Combustion and Thermochemistry PowerPoint Presentation, free What S The Meaning Of Heat Of Vaporization the heat of vaporization is equal to the thermal energy required for vaporization divided by the mass of the substance that is vaporizing. the heat of vaporization is the amount of energy required to convert or evaporate a specific amount of a substance from a. the heat (or enthalpy) of vaporization is the quantity of heat that. What S The Meaning Of Heat Of Vaporization.
From www.slideserve.com
PPT Liquids and Solids PowerPoint Presentation, free download ID What S The Meaning Of Heat Of Vaporization the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and. the heat of vaporization is the amount of energy required to convert or evaporate a specific amount of a substance from a. the molar heat of vaporization \(\left( \delta h_\text{vap}. What S The Meaning Of Heat Of Vaporization.
From education-portal.com
Heat of Vaporization Definition & Equation Video & Lesson Transcript What S The Meaning Of Heat Of Vaporization the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. the heat of vaporization is equal to the thermal energy required for vaporization divided by the mass of the substance that is vaporizing. the heat of vaporization is the quantity of heat that needs to be. What S The Meaning Of Heat Of Vaporization.
From www.slideserve.com
PPT Heat PowerPoint Presentation, free download ID5769198 What S The Meaning Of Heat Of Vaporization the heat of vaporization is the amount of energy required to convert or evaporate a specific amount of a substance from a. the heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the. What S The Meaning Of Heat Of Vaporization.
From www.slideserve.com
PPT Energy and Chemical Change PowerPoint Presentation ID1278045 What S The Meaning Of Heat Of Vaporization the heat of vaporization is the amount of energy required to convert or evaporate a specific amount of a substance from a. the heat of vaporization is the quantity of heat that needs to be absorbed to vaporize a particular quantity of liquid at a constant. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a. What S The Meaning Of Heat Of Vaporization.
From www.worksheetsplanet.com
What is Vaporization Definition of Vaporization What S The Meaning Of Heat Of Vaporization the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and. the heat of vaporization is the amount of energy required to convert or evaporate a specific amount of a substance from a. the heat (or enthalpy) of vaporization is the. What S The Meaning Of Heat Of Vaporization.
From testbook.com
Heat of Vapourization Formula Definition, Formula & Solved Examples What S The Meaning Of Heat Of Vaporization the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and. the heat of vaporization is equal to the thermal energy required for vaporization divided by the mass of the substance that is vaporizing. the heat of vaporization is the quantity. What S The Meaning Of Heat Of Vaporization.
From www.nachi.org
Latent Heat of Vaporization Inspection Gallery InterNACHI® What S The Meaning Of Heat Of Vaporization the heat of vaporization is the amount of energy required to convert or evaporate a specific amount of a substance from a. the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and. the heat of vaporization is the quantity of. What S The Meaning Of Heat Of Vaporization.
From www.pinterest.jp
Heat Of Vaporization Easy Science Easy science, Chemical changes What S The Meaning Of Heat Of Vaporization the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. the heat of vaporization is equal to the thermal energy required for vaporization divided by the mass of the substance that is vaporizing. the heat of vaporization is the amount of energy required to convert or. What S The Meaning Of Heat Of Vaporization.
From engineerexcel.com
Heat of Vaporization Explained EngineerExcel What S The Meaning Of Heat Of Vaporization the heat of vaporization is the amount of energy required to convert or evaporate a specific amount of a substance from a. the heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the. What S The Meaning Of Heat Of Vaporization.
From www.ck12.org
Heats of Vaporization and Condensation CK12 Foundation What S The Meaning Of Heat Of Vaporization the heat of vaporization is equal to the thermal energy required for vaporization divided by the mass of the substance that is vaporizing. the heat of vaporization is the amount of energy required to convert or evaporate a specific amount of a substance from a. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance. What S The Meaning Of Heat Of Vaporization.
From www.slideshare.net
Enthalpy of vaporization of liquid What S The Meaning Of Heat Of Vaporization the heat of vaporization is the quantity of heat that needs to be absorbed to vaporize a particular quantity of liquid at a constant. the heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid. the heat of vaporization is the amount of energy required to convert. What S The Meaning Of Heat Of Vaporization.
From www.slideserve.com
PPT Thermochemistry PowerPoint Presentation, free download ID2841621 What S The Meaning Of Heat Of Vaporization the heat of vaporization is the amount of energy required to convert or evaporate a specific amount of a substance from a. the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and. the molar heat of vaporization \(\left( \delta h_\text{vap}. What S The Meaning Of Heat Of Vaporization.
From material-properties.org
Latent Heat of Vaporization of Chemical Elements Material Properties What S The Meaning Of Heat Of Vaporization the heat of vaporization is equal to the thermal energy required for vaporization divided by the mass of the substance that is vaporizing. the heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is. What S The Meaning Of Heat Of Vaporization.
From www.ck12.org
Heat of vaporization Example 1 ( Video ) Chemistry CK12 Foundation What S The Meaning Of Heat Of Vaporization the heat of vaporization is the quantity of heat that needs to be absorbed to vaporize a particular quantity of liquid at a constant. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. the heat of vaporization is the enthalpy change when a unit mass. What S The Meaning Of Heat Of Vaporization.
From www.tec-science.com
Specific latent heat of vaporization tecscience What S The Meaning Of Heat Of Vaporization the heat of vaporization is the quantity of heat that needs to be absorbed to vaporize a particular quantity of liquid at a constant. the heat of vaporization is equal to the thermal energy required for vaporization divided by the mass of the substance that is vaporizing. the heat (or enthalpy) of vaporization is the quantity of. What S The Meaning Of Heat Of Vaporization.
From pubs.acs.org
Vapor Pressure and Heat of Vaporization of Molecules That Associate in What S The Meaning Of Heat Of Vaporization the heat of vaporization is equal to the thermal energy required for vaporization divided by the mass of the substance that is vaporizing. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. the heat of vaporization is the quantity of heat that needs to be. What S The Meaning Of Heat Of Vaporization.
From www.tec-science.com
Specific latent heat of condensation tecscience What S The Meaning Of Heat Of Vaporization the heat of vaporization is the quantity of heat that needs to be absorbed to vaporize a particular quantity of liquid at a constant. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. the heat of vaporization is equal to the thermal energy required for. What S The Meaning Of Heat Of Vaporization.
From worksheetcampusbrows.z13.web.core.windows.net
Heat Of Fusion And Heat Of Vaporization What S The Meaning Of Heat Of Vaporization the heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid. the heat of vaporization is the amount of energy required to convert or evaporate a specific amount of a substance from a. the heat of vaporization is the quantity of heat that needs to be absorbed. What S The Meaning Of Heat Of Vaporization.
From worksheetcampusbrows.z13.web.core.windows.net
Heat Of Vaporization Chart What S The Meaning Of Heat Of Vaporization the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. the heat of vaporization is the amount of energy required to convert or evaporate a specific amount of a substance from a. the heat of vaporization is equal to the thermal energy required for vaporization divided. What S The Meaning Of Heat Of Vaporization.
From www.slideserve.com
PPT The Extraordinary Properties of Water PowerPoint Presentation What S The Meaning Of Heat Of Vaporization the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\) of a substance is the heat absorbed by one mole of that. the heat of vaporization is the amount of energy required to convert or evaporate a specific amount of a substance from a. the heat of vaporization is the quantity of heat that needs to be absorbed. What S The Meaning Of Heat Of Vaporization.
From learningschoolandy.z21.web.core.windows.net
Heat Of Vaporization And Fusion What S The Meaning Of Heat Of Vaporization the heat of vaporization is the enthalpy change when a unit mass of a substance changes its state from liquid to gas at a constant temperature and. the heat (or enthalpy) of vaporization is the quantity of heat that must be absorbed if a certain quantity of liquid. the molar heat of vaporization \(\left( \delta h_\text{vap} \right)\). What S The Meaning Of Heat Of Vaporization.
From www.slideserve.com
PPT Unit 4 The Hydrosphere PowerPoint Presentation, free download What S The Meaning Of Heat Of Vaporization the heat of vaporization is equal to the thermal energy required for vaporization divided by the mass of the substance that is vaporizing. the heat of vaporization is the amount of energy required to convert or evaporate a specific amount of a substance from a. the heat of vaporization is the quantity of heat that needs to. What S The Meaning Of Heat Of Vaporization.