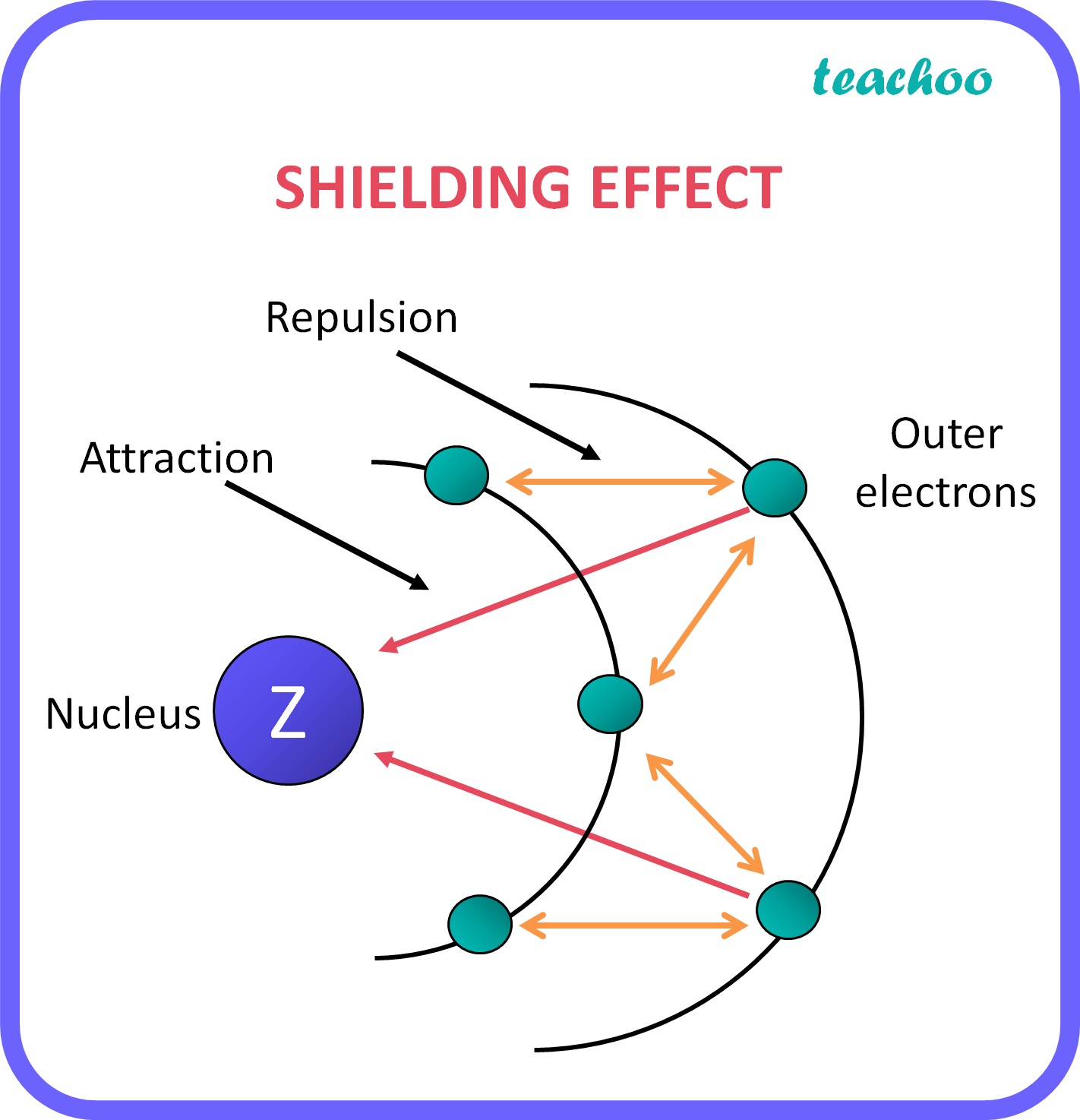
How Does Electron Shielding Work . Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. Hence, the nucleus has less grip on the outer electrons and are shielded from them.
from ar.inspiredpencil.com
Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and.
Electron Shielding
How Does Electron Shielding Work Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and.
From www.youtube.com
nuclear charge and electron shielding YouTube How Does Electron Shielding Work Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. How Does Electron Shielding Work.
From general.chemistrysteps.com
Ionization energy Chemistry Steps How Does Electron Shielding Work Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. How Does Electron Shielding Work.
From ar.inspiredpencil.com
Electron Shielding How Does Electron Shielding Work Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded from them. How Does Electron Shielding Work.
From www.slideserve.com
PPT • Chromatography (Separations) • Mass Spectrometry • Infrared (IR How Does Electron Shielding Work Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. How Does Electron Shielding Work.
From www.slideserve.com
PPT Lesson objectives • Define first ionisation energy and successive How Does Electron Shielding Work Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. How Does Electron Shielding Work.
From www.youtube.com
Electron shielding YouTube How Does Electron Shielding Work Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. Hence, the nucleus has less grip on the outer electrons and are shielded from them. How Does Electron Shielding Work.
From surfguppy.com
What is Electronegativity? How Does Electron Shielding Work Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. How Does Electron Shielding Work.
From www.showme.com
Electron shielding Science, Chemistry, Electrons, electron shielding How Does Electron Shielding Work Hence, the nucleus has less grip on the outer electrons and are shielded from them. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. How Does Electron Shielding Work.
From localrevive.com
What Is It? How Does It Work? Materials (2022) How Does Electron Shielding Work Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. Hence, the nucleus has less grip on the outer electrons and are shielded from them. How Does Electron Shielding Work.
From www.slideserve.com
PPT Unit 2 20142015 PowerPoint Presentation, free download ID How Does Electron Shielding Work Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. How Does Electron Shielding Work.
From slidesharetrick.blogspot.com
What Is Electron Shielding slidesharetrick How Does Electron Shielding Work Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. How Does Electron Shielding Work.
From byjus.com
What is shielding and deshielding in NMR? Give an example? How Does Electron Shielding Work Hence, the nucleus has less grip on the outer electrons and are shielded from them. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. How Does Electron Shielding Work.
From www.slideserve.com
PPT Chapter 14 Chemical Periodicity PowerPoint Presentation, free How Does Electron Shielding Work Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. How Does Electron Shielding Work.
From ar.inspiredpencil.com
Shielding Effect Electrons How Does Electron Shielding Work Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded from them. How Does Electron Shielding Work.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID5583827 How Does Electron Shielding Work Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. How Does Electron Shielding Work.
From chemistry.stackexchange.com
chemistry My book's claim about the shielding effect of s,p How Does Electron Shielding Work Hence, the nucleus has less grip on the outer electrons and are shielded from them. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. How Does Electron Shielding Work.
From www.youtube.com
S3.1.3 Electron shielding and effective nuclear charge YouTube How Does Electron Shielding Work Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. Hence, the nucleus has less grip on the outer electrons and are shielded from them. How Does Electron Shielding Work.
From socratic.org
How are shielding effect and atomic radius related? Socratic How Does Electron Shielding Work Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded from them. How Does Electron Shielding Work.
From slideplayer.com
Coulomb’s Law PERIODIC TRENDS Nuclear Charge Electron Shielding ppt How Does Electron Shielding Work Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. Hence, the nucleus has less grip on the outer electrons and are shielded from them. How Does Electron Shielding Work.
From simpleenglishchemistry.blogspot.com
Simple English Chemistry Atomic Size/Atomic Radius, Electronegativity How Does Electron Shielding Work Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. How Does Electron Shielding Work.
From ar.inspiredpencil.com
Shielding Effect Electrons How Does Electron Shielding Work Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. How Does Electron Shielding Work.
From slideplayer.com
Periodic Trends Chemistry I. ppt download How Does Electron Shielding Work Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. How Does Electron Shielding Work.
From www.slideserve.com
PPT The Periodic Table and Physical Properties PowerPoint How Does Electron Shielding Work Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. How Does Electron Shielding Work.
From byjus.com
does the shield that is developed by the inner electrons except the How Does Electron Shielding Work Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. Hence, the nucleus has less grip on the outer electrons and are shielded from them. How Does Electron Shielding Work.
From www.youtube.com
Shielding Effect and Effective Nuclear Charge YouTube How Does Electron Shielding Work Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded from them. How Does Electron Shielding Work.
From wisc.pb.unizin.org
Core and Valence Electrons, Shielding, Zeff (M7Q8) UWMadison How Does Electron Shielding Work Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded from them. How Does Electron Shielding Work.
From www.youtube.com
Shielding Deshielding Effects Explained Nucleus Get better grade in How Does Electron Shielding Work Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. How Does Electron Shielding Work.
From chem.libretexts.org
3.2 Shielding Chemistry LibreTexts How Does Electron Shielding Work Hence, the nucleus has less grip on the outer electrons and are shielded from them. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. How Does Electron Shielding Work.
From www.pearson.com
How does electron shielding in multielectron atoms give rise to e How Does Electron Shielding Work Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded from them. How Does Electron Shielding Work.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID1588021 How Does Electron Shielding Work Hence, the nucleus has less grip on the outer electrons and are shielded from them. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. How Does Electron Shielding Work.
From www.pearson.com
How does electron shielding in multielectron atoms give rise to e How Does Electron Shielding Work Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Hence, the nucleus has less grip on the outer electrons and are shielded from them. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. How Does Electron Shielding Work.
From www.numerade.com
SOLVEDWhat is shielding? In an atom, which electrons tend to do the How Does Electron Shielding Work Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. Hence, the nucleus has less grip on the outer electrons and are shielded from them. How Does Electron Shielding Work.
From slideplayer.com
Periodic Trends. ppt download How Does Electron Shielding Work Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. Hence, the nucleus has less grip on the outer electrons and are shielded from them. How Does Electron Shielding Work.
From slidesharetrick.blogspot.com
What Is Electron Shielding slidesharetrick How Does Electron Shielding Work Hence, the nucleus has less grip on the outer electrons and are shielded from them. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. How Does Electron Shielding Work.
From www.youtube.com
Shielding and Deshielding H NMR Spectroscopy YouTube How Does Electron Shielding Work Shielding refers to the core electrons repelling the outer rings and thus lowering the 1:1 ratio. Higher levels of shielding result in a reduced attraction between the outermost electrons and the nucleus, leading to larger atomic size and. Hence, the nucleus has less grip on the outer electrons and are shielded from them. How Does Electron Shielding Work.