Chlorine And Bromine Are Kept In A Same Group . Bromine and chlorine are chemical elements in the halogen group and are commonly used as disinfectants in swimming pools and spas. Includes trends in atomic and physical properties, the. Chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. Learn about the properties and uses of chlorine, bromine and iodine, the three common halogens. They have similar physical properties, such as boiling point, melting point. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). As chlorine and bromine consists of the same number of valence electrons in the outermost shell so they are kept in the same. Covers the halogens in group 17: The fall in reactivity is because the halogens become less good oxidising agents as you go.
from www.examples.com
They have similar physical properties, such as boiling point, melting point. Includes trends in atomic and physical properties, the. As chlorine and bromine consists of the same number of valence electrons in the outermost shell so they are kept in the same. Covers the halogens in group 17: Bromine and chlorine are chemical elements in the halogen group and are commonly used as disinfectants in swimming pools and spas. Learn about the properties and uses of chlorine, bromine and iodine, the three common halogens. The fall in reactivity is because the halogens become less good oxidising agents as you go. Chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. Fluorine (f), chlorine (cl), bromine (br) and iodine (i).
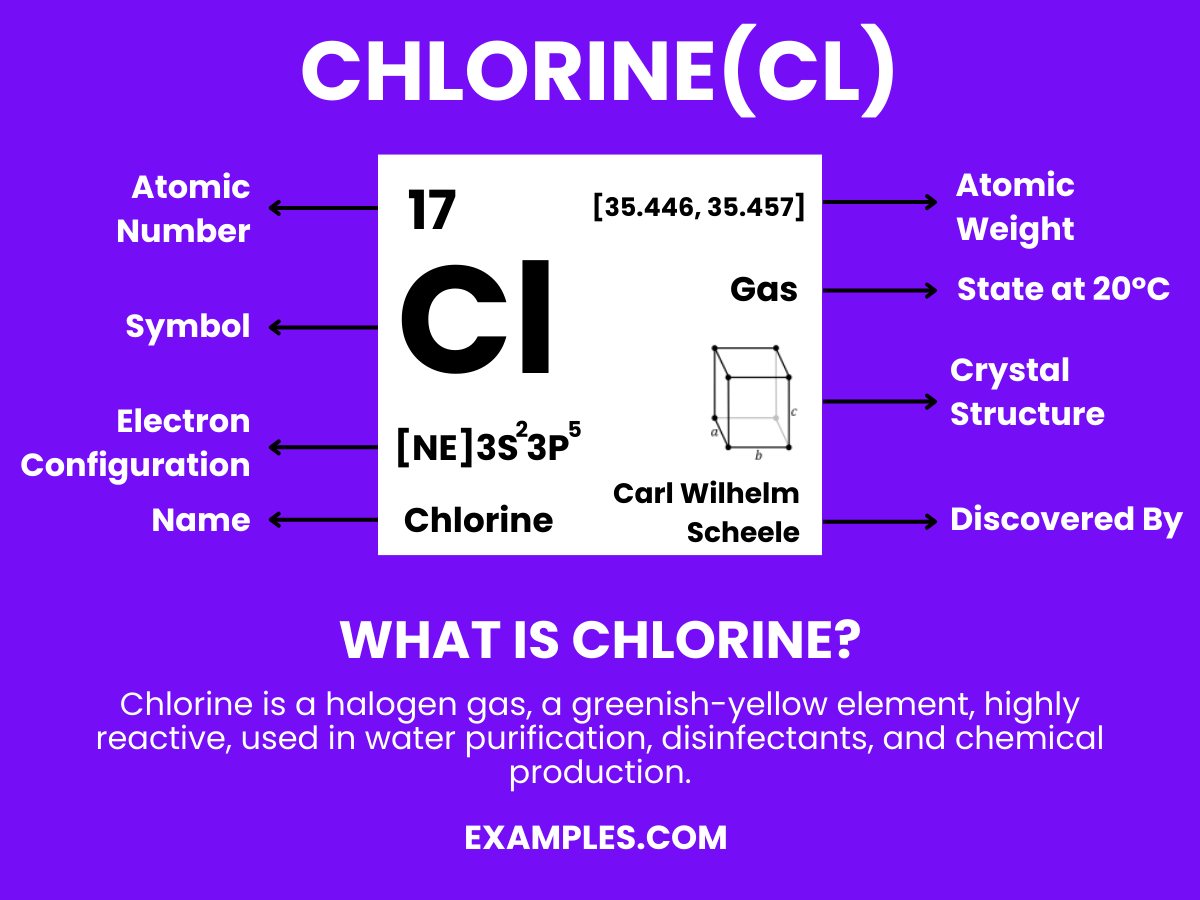
Chlorine (Cl) Definition, Preparation, Properties, Uses, Compounds
Chlorine And Bromine Are Kept In A Same Group Bromine and chlorine are chemical elements in the halogen group and are commonly used as disinfectants in swimming pools and spas. Learn about the properties and uses of chlorine, bromine and iodine, the three common halogens. The fall in reactivity is because the halogens become less good oxidising agents as you go. Includes trends in atomic and physical properties, the. Covers the halogens in group 17: They have similar physical properties, such as boiling point, melting point. As chlorine and bromine consists of the same number of valence electrons in the outermost shell so they are kept in the same. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). Chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. Bromine and chlorine are chemical elements in the halogen group and are commonly used as disinfectants in swimming pools and spas.
From www.myxxgirl.com
Analysis Of Chlorine Bromine And Iodine In Water Using Icp Oes Horiba Chlorine And Bromine Are Kept In A Same Group Chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. As chlorine and bromine consists of the same number of valence electrons in the outermost shell so they are kept in the same. They have similar physical properties, such as boiling point, melting point. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). Covers the halogens in. Chlorine And Bromine Are Kept In A Same Group.
From www.reddit.com
Excessive bromine and chlorine in tap water r/water Chlorine And Bromine Are Kept In A Same Group Learn about the properties and uses of chlorine, bromine and iodine, the three common halogens. Bromine and chlorine are chemical elements in the halogen group and are commonly used as disinfectants in swimming pools and spas. The fall in reactivity is because the halogens become less good oxidising agents as you go. They have similar physical properties, such as boiling. Chlorine And Bromine Are Kept In A Same Group.
From www.quimipool.com
Chlorine and bromine tablet dispenser for swimming pools Chlorine And Bromine Are Kept In A Same Group Learn about the properties and uses of chlorine, bromine and iodine, the three common halogens. Covers the halogens in group 17: As chlorine and bromine consists of the same number of valence electrons in the outermost shell so they are kept in the same. Includes trends in atomic and physical properties, the. Bromine and chlorine are chemical elements in the. Chlorine And Bromine Are Kept In A Same Group.
From www.semanticscholar.org
Figure 11 from Disinfection Efficiency and Relative Toxicity of Chlorine And Bromine Are Kept In A Same Group Bromine and chlorine are chemical elements in the halogen group and are commonly used as disinfectants in swimming pools and spas. The fall in reactivity is because the halogens become less good oxidising agents as you go. As chlorine and bromine consists of the same number of valence electrons in the outermost shell so they are kept in the same.. Chlorine And Bromine Are Kept In A Same Group.
From www.researchgate.net
(PDF) Effect of chlorine and bromine on the optical Chlorine And Bromine Are Kept In A Same Group Bromine and chlorine are chemical elements in the halogen group and are commonly used as disinfectants in swimming pools and spas. Covers the halogens in group 17: Learn about the properties and uses of chlorine, bromine and iodine, the three common halogens. They have similar physical properties, such as boiling point, melting point. Fluorine (f), chlorine (cl), bromine (br) and. Chlorine And Bromine Are Kept In A Same Group.
From www.pinterest.com
Liquid Chlorine or Chlorine Granules? in 2023 Pool maintenance Chlorine And Bromine Are Kept In A Same Group Bromine and chlorine are chemical elements in the halogen group and are commonly used as disinfectants in swimming pools and spas. Includes trends in atomic and physical properties, the. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). Learn about the properties and uses of chlorine, bromine and iodine, the three common halogens. Covers the halogens in group 17: As. Chlorine And Bromine Are Kept In A Same Group.
From spasearch.org
Chlorine vs. Bromine Which is Best for Your Home Spa? Chlorine And Bromine Are Kept In A Same Group Chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. Covers the halogens in group 17: The fall in reactivity is because the halogens become less good oxidising agents as you go. They have similar physical properties, such as boiling point, melting point. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). Includes trends in atomic and. Chlorine And Bromine Are Kept In A Same Group.
From askanydifference.com
Bromine vs Chlorine Difference and Comparison Chlorine And Bromine Are Kept In A Same Group They have similar physical properties, such as boiling point, melting point. Bromine and chlorine are chemical elements in the halogen group and are commonly used as disinfectants in swimming pools and spas. Covers the halogens in group 17: Learn about the properties and uses of chlorine, bromine and iodine, the three common halogens. As chlorine and bromine consists of the. Chlorine And Bromine Are Kept In A Same Group.
From www.semanticscholar.org
Figure 1 from Determination of total chlorine and bromine in solid Chlorine And Bromine Are Kept In A Same Group They have similar physical properties, such as boiling point, melting point. Includes trends in atomic and physical properties, the. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). Learn about the properties and uses of chlorine, bromine and iodine, the three common halogens. Chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. The fall in reactivity. Chlorine And Bromine Are Kept In A Same Group.
From www.manualowl.com
Pentair Automatic Chlorine and Bromine Feeders Chlorine Bromine High Chlorine And Bromine Are Kept In A Same Group As chlorine and bromine consists of the same number of valence electrons in the outermost shell so they are kept in the same. Includes trends in atomic and physical properties, the. They have similar physical properties, such as boiling point, melting point. Learn about the properties and uses of chlorine, bromine and iodine, the three common halogens. Fluorine (f), chlorine. Chlorine And Bromine Are Kept In A Same Group.
From poolblue.com
Pool Bromine vs. Chlorine What's the Difference? Poolblue Chlorine And Bromine Are Kept In A Same Group The fall in reactivity is because the halogens become less good oxidising agents as you go. They have similar physical properties, such as boiling point, melting point. Bromine and chlorine are chemical elements in the halogen group and are commonly used as disinfectants in swimming pools and spas. As chlorine and bromine consists of the same number of valence electrons. Chlorine And Bromine Are Kept In A Same Group.
From www.numerade.com
SOLVED Chlorine and bromine react In the dark WIth alkenes The Chlorine And Bromine Are Kept In A Same Group Covers the halogens in group 17: The fall in reactivity is because the halogens become less good oxidising agents as you go. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). They have similar physical properties, such as boiling point, melting point. As chlorine and bromine consists of the same number of valence electrons in the outermost shell so they. Chlorine And Bromine Are Kept In A Same Group.
From darlly.eu
What is the difference between chlorine and bromine? Chlorine And Bromine Are Kept In A Same Group Includes trends in atomic and physical properties, the. The fall in reactivity is because the halogens become less good oxidising agents as you go. They have similar physical properties, such as boiling point, melting point. Chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. Bromine and chlorine are chemical elements in the halogen group and are. Chlorine And Bromine Are Kept In A Same Group.
From blog.chloramineconsulting.com
Chlorine vs. Bromine in Indoor Pools Chlorine And Bromine Are Kept In A Same Group Covers the halogens in group 17: They have similar physical properties, such as boiling point, melting point. Includes trends in atomic and physical properties, the. Learn about the properties and uses of chlorine, bromine and iodine, the three common halogens. Chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. Fluorine (f), chlorine (cl), bromine (br) and. Chlorine And Bromine Are Kept In A Same Group.
From www.examples.com
Chlorine (Cl) Definition, Preparation, Properties, Uses, Compounds Chlorine And Bromine Are Kept In A Same Group They have similar physical properties, such as boiling point, melting point. Chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). Bromine and chlorine are chemical elements in the halogen group and are commonly used as disinfectants in swimming pools and spas. The fall in reactivity is because. Chlorine And Bromine Are Kept In A Same Group.
From www.poolpondspaonline.co.za
POOL CHLORINE BIOGUARD BIO CHLOR‘Plus’ 3.5KG PPS Online Chlorine And Bromine Are Kept In A Same Group Bromine and chlorine are chemical elements in the halogen group and are commonly used as disinfectants in swimming pools and spas. The fall in reactivity is because the halogens become less good oxidising agents as you go. Chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. They have similar physical properties, such as boiling point, melting. Chlorine And Bromine Are Kept In A Same Group.
From serumwatercare.com
Bromine vs Chlorine In A Hot Tub? Serum Watercare Chlorine And Bromine Are Kept In A Same Group As chlorine and bromine consists of the same number of valence electrons in the outermost shell so they are kept in the same. Covers the halogens in group 17: They have similar physical properties, such as boiling point, melting point. Chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. Fluorine (f), chlorine (cl), bromine (br) and. Chlorine And Bromine Are Kept In A Same Group.
From askanydifference.com
Bromine vs Chlorine Difference and Comparison Chlorine And Bromine Are Kept In A Same Group Learn about the properties and uses of chlorine, bromine and iodine, the three common halogens. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). Bromine and chlorine are chemical elements in the halogen group and are commonly used as disinfectants in swimming pools and spas. As chlorine and bromine consists of the same number of valence electrons in the outermost. Chlorine And Bromine Are Kept In A Same Group.
From www.piscine-sav.com
Chlorine and bromine neutralizer for swimming pools 5 L canister 55... Chlorine And Bromine Are Kept In A Same Group The fall in reactivity is because the halogens become less good oxidising agents as you go. Chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. Covers the halogens in group 17: Fluorine (f), chlorine (cl), bromine (br) and iodine (i). As chlorine and bromine consists of the same number of valence electrons in the outermost shell. Chlorine And Bromine Are Kept In A Same Group.
From snl.no
brom Store norske leksikon Chlorine And Bromine Are Kept In A Same Group The fall in reactivity is because the halogens become less good oxidising agents as you go. Learn about the properties and uses of chlorine, bromine and iodine, the three common halogens. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). They have similar physical properties, such as boiling point, melting point. Includes trends in atomic and physical properties, the. Chlorine. Chlorine And Bromine Are Kept In A Same Group.
From www.masterspas.com
Chlorine or bromine? Choosing the best hot tub sanitizer Master Spas Blog Chlorine And Bromine Are Kept In A Same Group Fluorine (f), chlorine (cl), bromine (br) and iodine (i). Covers the halogens in group 17: Bromine and chlorine are chemical elements in the halogen group and are commonly used as disinfectants in swimming pools and spas. Chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. Learn about the properties and uses of chlorine, bromine and iodine,. Chlorine And Bromine Are Kept In A Same Group.
From www.chegg.com
Solved Chlorine and bromine react in the dark with alkenes. Chlorine And Bromine Are Kept In A Same Group Includes trends in atomic and physical properties, the. Learn about the properties and uses of chlorine, bromine and iodine, the three common halogens. Chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. The fall in reactivity is because the halogens become less good oxidising agents as you go. As chlorine and bromine consists of the same. Chlorine And Bromine Are Kept In A Same Group.
From www.greatbayspas.com
Chlorine Vs Bromine Great Bay Spa & Sauna Chlorine And Bromine Are Kept In A Same Group The fall in reactivity is because the halogens become less good oxidising agents as you go. They have similar physical properties, such as boiling point, melting point. As chlorine and bromine consists of the same number of valence electrons in the outermost shell so they are kept in the same. Chlorine and bromine produce iron(iii) chloride or bromide, but iodine. Chlorine And Bromine Are Kept In A Same Group.
From solvedlib.com
Chlorine and bromine react in the dark with alkenes. … SolvedLib Chlorine And Bromine Are Kept In A Same Group Includes trends in atomic and physical properties, the. Chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. The fall in reactivity is because the halogens become less good oxidising agents as you go. Learn about the properties and uses of chlorine, bromine and iodine, the three common halogens. They have similar physical properties, such as boiling. Chlorine And Bromine Are Kept In A Same Group.
From www.quimipool.com
Inline chlorine and bromine dosing unit 4 kgs. Chlorine And Bromine Are Kept In A Same Group Chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. The fall in reactivity is because the halogens become less good oxidising agents as you go. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). As chlorine and bromine consists of the same number of valence electrons in the outermost shell so they are kept in the. Chlorine And Bromine Are Kept In A Same Group.
From www.numerade.com
SOLVED Aqueous sodium chloride (NaCl) and liquid bromine (Brz are Chlorine And Bromine Are Kept In A Same Group As chlorine and bromine consists of the same number of valence electrons in the outermost shell so they are kept in the same. They have similar physical properties, such as boiling point, melting point. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). Chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. Learn about the properties. Chlorine And Bromine Are Kept In A Same Group.
From martlabpro.com
Bromine Vs Chlorine For Skin Which Is Better? MartLabPro Chlorine And Bromine Are Kept In A Same Group As chlorine and bromine consists of the same number of valence electrons in the outermost shell so they are kept in the same. The fall in reactivity is because the halogens become less good oxidising agents as you go. Learn about the properties and uses of chlorine, bromine and iodine, the three common halogens. Includes trends in atomic and physical. Chlorine And Bromine Are Kept In A Same Group.
From dutchbarnlandscaping.com
Leisure Time Spa and Hot Tub Test Strips Dutch Barn Landscaping & Pools Chlorine And Bromine Are Kept In A Same Group Fluorine (f), chlorine (cl), bromine (br) and iodine (i). Bromine and chlorine are chemical elements in the halogen group and are commonly used as disinfectants in swimming pools and spas. Covers the halogens in group 17: Includes trends in atomic and physical properties, the. Chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. They have similar. Chlorine And Bromine Are Kept In A Same Group.
From www.ramirezpoolandspa.com
Chlorine & Bromine Test Drops Ramirez Pool & Spa Chlorine And Bromine Are Kept In A Same Group Covers the halogens in group 17: The fall in reactivity is because the halogens become less good oxidising agents as you go. Chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. Bromine and chlorine are chemical elements in the halogen group and are commonly used as disinfectants in swimming pools and spas. They have similar physical. Chlorine And Bromine Are Kept In A Same Group.
From www.researchgate.net
(PDF) Measurements of photolyzable chlorine and bromine during the Chlorine And Bromine Are Kept In A Same Group Covers the halogens in group 17: The fall in reactivity is because the halogens become less good oxidising agents as you go. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). Learn about the properties and uses of chlorine, bromine and iodine, the three common halogens. As chlorine and bromine consists of the same number of valence electrons in the. Chlorine And Bromine Are Kept In A Same Group.
From www.linkedin.com
Chlorine vs. Bromine Chlorine And Bromine Are Kept In A Same Group Chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. As chlorine and bromine consists of the same number of valence electrons in the outermost shell so they are kept in the same. Bromine and chlorine are chemical elements in the halogen group and are commonly used as disinfectants in swimming pools and spas. The fall in. Chlorine And Bromine Are Kept In A Same Group.
From tinyhousegarage.com
To Disinfect Swimming Pool Water We Use Bromine? Chlorine And Bromine Are Kept In A Same Group They have similar physical properties, such as boiling point, melting point. Includes trends in atomic and physical properties, the. The fall in reactivity is because the halogens become less good oxidising agents as you go. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). Covers the halogens in group 17: Bromine and chlorine are chemical elements in the halogen group. Chlorine And Bromine Are Kept In A Same Group.
From www.youtube.com
Difference Between Bromine and Chlorine YouTube Chlorine And Bromine Are Kept In A Same Group Bromine and chlorine are chemical elements in the halogen group and are commonly used as disinfectants in swimming pools and spas. Covers the halogens in group 17: Chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. Learn about the properties and uses of chlorine, bromine and iodine, the three common halogens. As chlorine and bromine consists. Chlorine And Bromine Are Kept In A Same Group.
From www.chegg.com
Solved Chlorine and bromine gas are kept in identical Chlorine And Bromine Are Kept In A Same Group Bromine and chlorine are chemical elements in the halogen group and are commonly used as disinfectants in swimming pools and spas. Fluorine (f), chlorine (cl), bromine (br) and iodine (i). They have similar physical properties, such as boiling point, melting point. Chlorine and bromine produce iron(iii) chloride or bromide, but iodine produces iron(ii) iodide. Covers the halogens in group 17:. Chlorine And Bromine Are Kept In A Same Group.
From brainly.com
Balance the chemical equation. Based on the equation, how many grams of Chlorine And Bromine Are Kept In A Same Group Fluorine (f), chlorine (cl), bromine (br) and iodine (i). Covers the halogens in group 17: They have similar physical properties, such as boiling point, melting point. As chlorine and bromine consists of the same number of valence electrons in the outermost shell so they are kept in the same. Includes trends in atomic and physical properties, the. Learn about the. Chlorine And Bromine Are Kept In A Same Group.