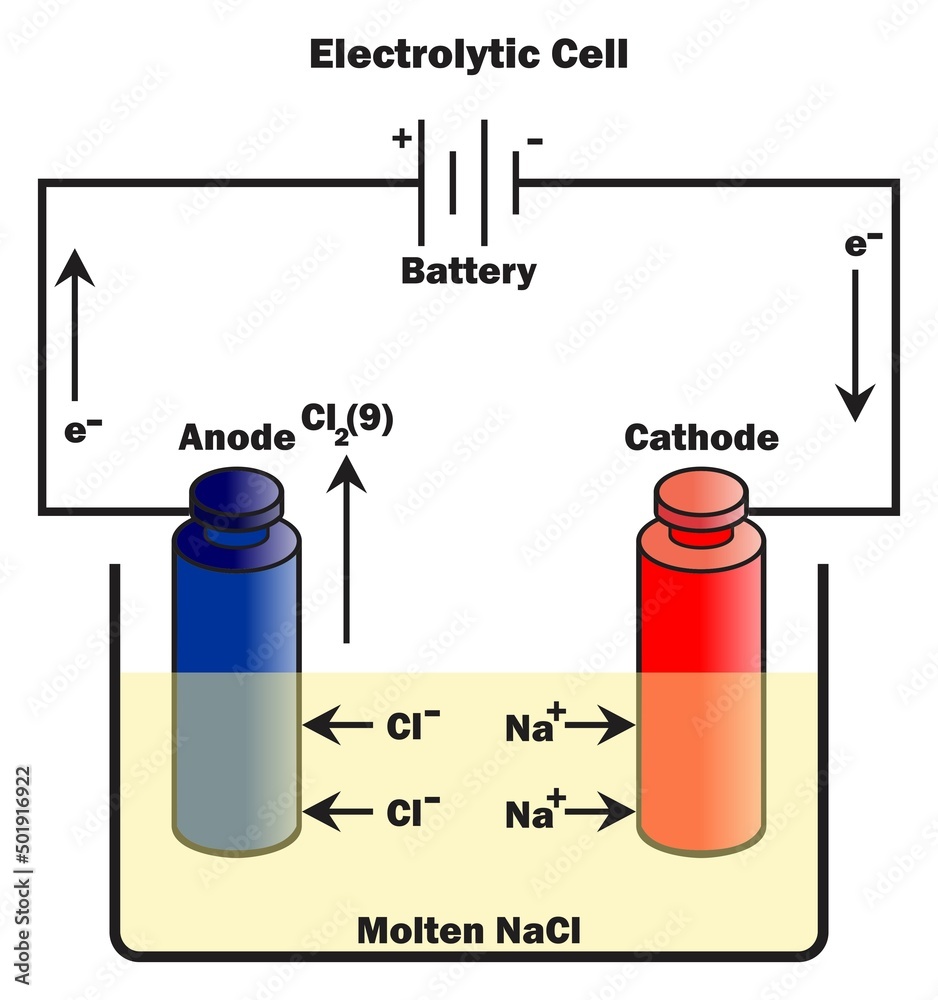
Positive Electrode In Electrolytic Reduction . The anode is the negative electrode at which oxidation occurs. Electrons from the negative terminal travel to the cathode and are used to reduce sodium ions into sodium atoms. If electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of the right electrode. Electrochemical co2 reduction (co2r) in acidic electrolytes has gained significant attention owing to higher carbon. Here the electrode sign is not being determined by the cell reaction, but by the external power. The electrolytic reduction method is to reduce eu 3 + into eu 2 + on cathode to separate eu 2 + and re 3 +, and there are three kinds of methods:. The cathode is the positive electrode at which reduction occurs. In an electrolytic cell this is the positive electrode. If molten nacl(l) n a c l (l) is placed into the container and inert electrodes of c(s) c (s) are inserted, attached to the positive and negative terminals of a battery, an electrolytic reaction will occur.
from stock.adobe.com
The electrolytic reduction method is to reduce eu 3 + into eu 2 + on cathode to separate eu 2 + and re 3 +, and there are three kinds of methods:. Electrochemical co2 reduction (co2r) in acidic electrolytes has gained significant attention owing to higher carbon. Here the electrode sign is not being determined by the cell reaction, but by the external power. The cathode is the positive electrode at which reduction occurs. If molten nacl(l) n a c l (l) is placed into the container and inert electrodes of c(s) c (s) are inserted, attached to the positive and negative terminals of a battery, an electrolytic reaction will occur. Electrons from the negative terminal travel to the cathode and are used to reduce sodium ions into sodium atoms. If electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of the right electrode. The anode is the negative electrode at which oxidation occurs. In an electrolytic cell this is the positive electrode.
Electrolytic cell infographic diagram with components including anode
Positive Electrode In Electrolytic Reduction The cathode is the positive electrode at which reduction occurs. The electrolytic reduction method is to reduce eu 3 + into eu 2 + on cathode to separate eu 2 + and re 3 +, and there are three kinds of methods:. Here the electrode sign is not being determined by the cell reaction, but by the external power. The cathode is the positive electrode at which reduction occurs. In an electrolytic cell this is the positive electrode. The anode is the negative electrode at which oxidation occurs. If electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of the right electrode. Electrochemical co2 reduction (co2r) in acidic electrolytes has gained significant attention owing to higher carbon. Electrons from the negative terminal travel to the cathode and are used to reduce sodium ions into sodium atoms. If molten nacl(l) n a c l (l) is placed into the container and inert electrodes of c(s) c (s) are inserted, attached to the positive and negative terminals of a battery, an electrolytic reaction will occur.
From chem.libretexts.org
9.4 Standard Electrode Potentials Chemistry LibreTexts Positive Electrode In Electrolytic Reduction The anode is the negative electrode at which oxidation occurs. If electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of the right electrode. In an electrolytic cell this is the positive electrode. If molten nacl(l) n a c l (l) is. Positive Electrode In Electrolytic Reduction.
From www.wattalps.com
Positive electrode the different technologies for liion battery Positive Electrode In Electrolytic Reduction If electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of the right electrode. Electrons from the negative terminal travel to the cathode and are used to reduce sodium ions into sodium atoms. The anode is the negative electrode at which oxidation. Positive Electrode In Electrolytic Reduction.
From www.numerade.com
SOLVED What is correct in an electrolytic cell? A B Electrode Anode Positive Electrode In Electrolytic Reduction Electrochemical co2 reduction (co2r) in acidic electrolytes has gained significant attention owing to higher carbon. If molten nacl(l) n a c l (l) is placed into the container and inert electrodes of c(s) c (s) are inserted, attached to the positive and negative terminals of a battery, an electrolytic reaction will occur. The electrolytic reduction method is to reduce eu. Positive Electrode In Electrolytic Reduction.
From stock.adobe.com
Ions movement to negative electrode and positive electrode Stock Photo Positive Electrode In Electrolytic Reduction If electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of the right electrode. Here the electrode sign is not being determined by the cell reaction, but by the external power. The anode is the negative electrode at which oxidation occurs. Electrochemical. Positive Electrode In Electrolytic Reduction.
From nl.pinterest.com
Electrochemical Cells STEM Physical Science Pinterest Brandweer Positive Electrode In Electrolytic Reduction Here the electrode sign is not being determined by the cell reaction, but by the external power. The anode is the negative electrode at which oxidation occurs. The cathode is the positive electrode at which reduction occurs. If electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in. Positive Electrode In Electrolytic Reduction.
From www.alamy.com
Ions movement to negative electrode and positive electrode. 3D Positive Electrode In Electrolytic Reduction If electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of the right electrode. The cathode is the positive electrode at which reduction occurs. The anode is the negative electrode at which oxidation occurs. In an electrolytic cell this is the positive. Positive Electrode In Electrolytic Reduction.
From www.sciencenewsforstudents.org
Explainer What is an electrode? Science News for Students Positive Electrode In Electrolytic Reduction In an electrolytic cell this is the positive electrode. The anode is the negative electrode at which oxidation occurs. The electrolytic reduction method is to reduce eu 3 + into eu 2 + on cathode to separate eu 2 + and re 3 +, and there are three kinds of methods:. Electrochemical co2 reduction (co2r) in acidic electrolytes has gained. Positive Electrode In Electrolytic Reduction.
From eureka.patsnap.com
Positive electrode active material as well as preparation method Positive Electrode In Electrolytic Reduction Here the electrode sign is not being determined by the cell reaction, but by the external power. Electrons from the negative terminal travel to the cathode and are used to reduce sodium ions into sodium atoms. In an electrolytic cell this is the positive electrode. Electrochemical co2 reduction (co2r) in acidic electrolytes has gained significant attention owing to higher carbon.. Positive Electrode In Electrolytic Reduction.
From uspto.report
Positive electrode active material precursor for lithium secondary Positive Electrode In Electrolytic Reduction Electrochemical co2 reduction (co2r) in acidic electrolytes has gained significant attention owing to higher carbon. Here the electrode sign is not being determined by the cell reaction, but by the external power. If molten nacl(l) n a c l (l) is placed into the container and inert electrodes of c(s) c (s) are inserted, attached to the positive and negative. Positive Electrode In Electrolytic Reduction.
From byjus.com
Where does reduction happen in electrolysis? Positive Electrode In Electrolytic Reduction If molten nacl(l) n a c l (l) is placed into the container and inert electrodes of c(s) c (s) are inserted, attached to the positive and negative terminals of a battery, an electrolytic reaction will occur. The electrolytic reduction method is to reduce eu 3 + into eu 2 + on cathode to separate eu 2 + and re. Positive Electrode In Electrolytic Reduction.
From www.coursehero.com
[Solved] The cathode of an electrolytic cell is the positive electrode Positive Electrode In Electrolytic Reduction The electrolytic reduction method is to reduce eu 3 + into eu 2 + on cathode to separate eu 2 + and re 3 +, and there are three kinds of methods:. Electrochemical co2 reduction (co2r) in acidic electrolytes has gained significant attention owing to higher carbon. If electrons flow from the left electrode to the right electrode (as depicted. Positive Electrode In Electrolytic Reduction.
From www.thebuzzevnews.com
New solidstate battery material could give big EV range boost Positive Electrode In Electrolytic Reduction If electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of the right electrode. The anode is the negative electrode at which oxidation occurs. If molten nacl(l) n a c l (l) is placed into the container and inert electrodes of c(s). Positive Electrode In Electrolytic Reduction.
From brainly.in
draw well labelled diagram of electrolytic cell used for electroplating Positive Electrode In Electrolytic Reduction If electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of the right electrode. In an electrolytic cell this is the positive electrode. The anode is the negative electrode at which oxidation occurs. The cathode is the positive electrode at which reduction. Positive Electrode In Electrolytic Reduction.
From www.theknowledgelibrary.in
Difference Between Cation & Anion The Knowledge Library Positive Electrode In Electrolytic Reduction The anode is the negative electrode at which oxidation occurs. If electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of the right electrode. Here the electrode sign is not being determined by the cell reaction, but by the external power. Electrochemical. Positive Electrode In Electrolytic Reduction.
From webmis.highland.cc.il.us
Electrolysis Positive Electrode In Electrolytic Reduction If electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of the right electrode. In an electrolytic cell this is the positive electrode. Here the electrode sign is not being determined by the cell reaction, but by the external power. The anode. Positive Electrode In Electrolytic Reduction.
From byjus.com
An electrolytic cell is used to convert Positive Electrode In Electrolytic Reduction The electrolytic reduction method is to reduce eu 3 + into eu 2 + on cathode to separate eu 2 + and re 3 +, and there are three kinds of methods:. If electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential. Positive Electrode In Electrolytic Reduction.
From anthropology.iresearchnet.com
Tool Parts ordinary electrolytic cell redox reaction electrolytic cell Positive Electrode In Electrolytic Reduction If molten nacl(l) n a c l (l) is placed into the container and inert electrodes of c(s) c (s) are inserted, attached to the positive and negative terminals of a battery, an electrolytic reaction will occur. The electrolytic reduction method is to reduce eu 3 + into eu 2 + on cathode to separate eu 2 + and re. Positive Electrode In Electrolytic Reduction.
From forumautomation.com
6 Differences between Anode and Cathode Electronics Industrial Positive Electrode In Electrolytic Reduction If electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of the right electrode. Electrons from the negative terminal travel to the cathode and are used to reduce sodium ions into sodium atoms. The cathode is the positive electrode at which reduction. Positive Electrode In Electrolytic Reduction.
From www.cleaverscientific.com
CSLIEF Replacement Spring positive electrode Cleaver Scientific Positive Electrode In Electrolytic Reduction The anode is the negative electrode at which oxidation occurs. The electrolytic reduction method is to reduce eu 3 + into eu 2 + on cathode to separate eu 2 + and re 3 +, and there are three kinds of methods:. If electrons flow from the left electrode to the right electrode (as depicted in the above cell notation). Positive Electrode In Electrolytic Reduction.
From eureka.patsnap.com
Schottky diode capable of switching positive electrode and negative Positive Electrode In Electrolytic Reduction If molten nacl(l) n a c l (l) is placed into the container and inert electrodes of c(s) c (s) are inserted, attached to the positive and negative terminals of a battery, an electrolytic reaction will occur. If electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in. Positive Electrode In Electrolytic Reduction.
From www.differencebetween.com
Difference Between Electrolytic Reduction and Refining Compare the Positive Electrode In Electrolytic Reduction If electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of the right electrode. In an electrolytic cell this is the positive electrode. Electrochemical co2 reduction (co2r) in acidic electrolytes has gained significant attention owing to higher carbon. The electrolytic reduction method. Positive Electrode In Electrolytic Reduction.
From ecopool.en.made-in-china.com
Positive and Negative Electrode Titanium Plate Electrolytic Salt Positive Electrode In Electrolytic Reduction Here the electrode sign is not being determined by the cell reaction, but by the external power. The cathode is the positive electrode at which reduction occurs. The anode is the negative electrode at which oxidation occurs. In an electrolytic cell this is the positive electrode. The electrolytic reduction method is to reduce eu 3 + into eu 2 +. Positive Electrode In Electrolytic Reduction.
From blog.naver.com
[독후감] 배터리전쟁 _ 안정과 관계 네이버 블로그 Positive Electrode In Electrolytic Reduction The anode is the negative electrode at which oxidation occurs. Here the electrode sign is not being determined by the cell reaction, but by the external power. The cathode is the positive electrode at which reduction occurs. In an electrolytic cell this is the positive electrode. Electrons from the negative terminal travel to the cathode and are used to reduce. Positive Electrode In Electrolytic Reduction.
From eureka.patsnap.com
Positive electrode material of alkali dry battery and preparation Positive Electrode In Electrolytic Reduction The anode is the negative electrode at which oxidation occurs. In an electrolytic cell this is the positive electrode. Electrons from the negative terminal travel to the cathode and are used to reduce sodium ions into sodium atoms. The cathode is the positive electrode at which reduction occurs. Here the electrode sign is not being determined by the cell reaction,. Positive Electrode In Electrolytic Reduction.
From www.nagwa.com
Question Video Recalling the Name of the Positive Electrode in an Positive Electrode In Electrolytic Reduction If electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of the right electrode. The cathode is the positive electrode at which reduction occurs. In an electrolytic cell this is the positive electrode. The electrolytic reduction method is to reduce eu 3. Positive Electrode In Electrolytic Reduction.
From dokumen.tips
(PDF) Positive electrode for galvanic cells prepared by anodization of Positive Electrode In Electrolytic Reduction Electrons from the negative terminal travel to the cathode and are used to reduce sodium ions into sodium atoms. Electrochemical co2 reduction (co2r) in acidic electrolytes has gained significant attention owing to higher carbon. If molten nacl(l) n a c l (l) is placed into the container and inert electrodes of c(s) c (s) are inserted, attached to the positive. Positive Electrode In Electrolytic Reduction.
From eureka.patsnap.com
Composition, preparation method and application in preparation of Positive Electrode In Electrolytic Reduction If molten nacl(l) n a c l (l) is placed into the container and inert electrodes of c(s) c (s) are inserted, attached to the positive and negative terminals of a battery, an electrolytic reaction will occur. The electrolytic reduction method is to reduce eu 3 + into eu 2 + on cathode to separate eu 2 + and re. Positive Electrode In Electrolytic Reduction.
From courses.lumenlearning.com
Standard Reduction Potentials Chemistry Atoms First Positive Electrode In Electrolytic Reduction The electrolytic reduction method is to reduce eu 3 + into eu 2 + on cathode to separate eu 2 + and re 3 +, and there are three kinds of methods:. Electrons from the negative terminal travel to the cathode and are used to reduce sodium ions into sodium atoms. The anode is the negative electrode at which oxidation. Positive Electrode In Electrolytic Reduction.
From stock.adobe.com
Electrolytic cell infographic diagram with components including anode Positive Electrode In Electrolytic Reduction In an electrolytic cell this is the positive electrode. Here the electrode sign is not being determined by the cell reaction, but by the external power. The cathode is the positive electrode at which reduction occurs. Electrochemical co2 reduction (co2r) in acidic electrolytes has gained significant attention owing to higher carbon. If electrons flow from the left electrode to the. Positive Electrode In Electrolytic Reduction.
From www.researchgate.net
Equivalent circuit diagram of the positive electrode of a leadacid Positive Electrode In Electrolytic Reduction The cathode is the positive electrode at which reduction occurs. Here the electrode sign is not being determined by the cell reaction, but by the external power. The electrolytic reduction method is to reduce eu 3 + into eu 2 + on cathode to separate eu 2 + and re 3 +, and there are three kinds of methods:. Electrochemical. Positive Electrode In Electrolytic Reduction.
From eureka.patsnap.com
Schottky diode capable of switching positive electrode and negative Positive Electrode In Electrolytic Reduction The electrolytic reduction method is to reduce eu 3 + into eu 2 + on cathode to separate eu 2 + and re 3 +, and there are three kinds of methods:. Electrochemical co2 reduction (co2r) in acidic electrolytes has gained significant attention owing to higher carbon. If electrons flow from the left electrode to the right electrode (as depicted. Positive Electrode In Electrolytic Reduction.
From www.pinterest.com
Anode positive in electro chemical ( cell ) Anode negative in electro Positive Electrode In Electrolytic Reduction If electrons flow from the left electrode to the right electrode (as depicted in the above cell notation) when the cell operates in its spontaneous direction, the potential of the right electrode. If molten nacl(l) n a c l (l) is placed into the container and inert electrodes of c(s) c (s) are inserted, attached to the positive and negative. Positive Electrode In Electrolytic Reduction.
From www.fdk.com
Positive electrode material │ FDK's original technology │ R&D │ FDK Positive Electrode In Electrolytic Reduction The electrolytic reduction method is to reduce eu 3 + into eu 2 + on cathode to separate eu 2 + and re 3 +, and there are three kinds of methods:. The cathode is the positive electrode at which reduction occurs. The anode is the negative electrode at which oxidation occurs. Electrochemical co2 reduction (co2r) in acidic electrolytes has. Positive Electrode In Electrolytic Reduction.
From brainly.com
1. Create a diagram of your electroplating apparatus (an electrolytic Positive Electrode In Electrolytic Reduction If molten nacl(l) n a c l (l) is placed into the container and inert electrodes of c(s) c (s) are inserted, attached to the positive and negative terminals of a battery, an electrolytic reaction will occur. Here the electrode sign is not being determined by the cell reaction, but by the external power. The cathode is the positive electrode. Positive Electrode In Electrolytic Reduction.
From eureka.patsnap.com
Method for intensifying leaching of active matter in positive electrode Positive Electrode In Electrolytic Reduction Electrochemical co2 reduction (co2r) in acidic electrolytes has gained significant attention owing to higher carbon. Electrons from the negative terminal travel to the cathode and are used to reduce sodium ions into sodium atoms. In an electrolytic cell this is the positive electrode. Here the electrode sign is not being determined by the cell reaction, but by the external power.. Positive Electrode In Electrolytic Reduction.