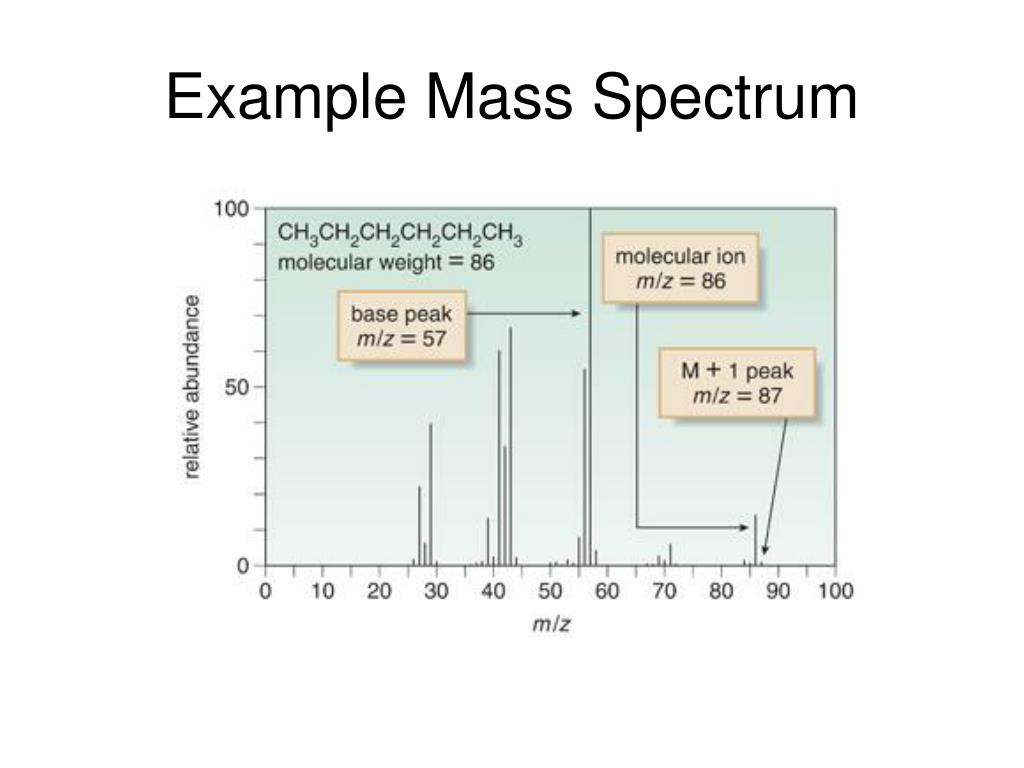
Mass Spectroscopy Example . The three essential functions of a mass spectrometer, and the associated components, are: Mass spectroscopy is the accurate method for determining of molecular mass of the compound and its elemental composition. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that they. This chapter provides an overview of mass spectrometry, focusing on ionization sources and their significance in the development of mass. C 5 h 12 has an exact mass of 72.0939 amu, whereas c 4 h 8 o has an exact mass of 72.0575 amu. If something is moving and you subject it to a sideways force, instead of moving in a straight. Mass spectrometry allows us to measure the masses of atoms and molecules, and also obtain information about their chemical structure. Before we talk about interpreting spectra, let’s. This page describes how a mass spectrum is produced using a mass spectrometer. When molecules are bombarded with an energetic electron beam,. A small sample is ionized, usually to cations by loss of an electron. For example, both c 5 h 12 and c 4 h 8 o have mw = 72, but they differ slightly beyond the decimal point:
from www.slideserve.com
The three essential functions of a mass spectrometer, and the associated components, are: Mass spectrometry allows us to measure the masses of atoms and molecules, and also obtain information about their chemical structure. Before we talk about interpreting spectra, let’s. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that they. A small sample is ionized, usually to cations by loss of an electron. For example, both c 5 h 12 and c 4 h 8 o have mw = 72, but they differ slightly beyond the decimal point: This chapter provides an overview of mass spectrometry, focusing on ionization sources and their significance in the development of mass. This page describes how a mass spectrum is produced using a mass spectrometer. When molecules are bombarded with an energetic electron beam,. Mass spectroscopy is the accurate method for determining of molecular mass of the compound and its elemental composition.
PPT Mass Spectrometry PowerPoint Presentation, free download ID6357895
Mass Spectroscopy Example Before we talk about interpreting spectra, let’s. For example, both c 5 h 12 and c 4 h 8 o have mw = 72, but they differ slightly beyond the decimal point: This chapter provides an overview of mass spectrometry, focusing on ionization sources and their significance in the development of mass. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that they. Before we talk about interpreting spectra, let’s. Mass spectrometry allows us to measure the masses of atoms and molecules, and also obtain information about their chemical structure. Mass spectroscopy is the accurate method for determining of molecular mass of the compound and its elemental composition. When molecules are bombarded with an energetic electron beam,. C 5 h 12 has an exact mass of 72.0939 amu, whereas c 4 h 8 o has an exact mass of 72.0575 amu. This page describes how a mass spectrum is produced using a mass spectrometer. The three essential functions of a mass spectrometer, and the associated components, are: A small sample is ionized, usually to cations by loss of an electron. If something is moving and you subject it to a sideways force, instead of moving in a straight.
From www.studypool.com
SOLUTION Mass spectroscopy examples Studypool Mass Spectroscopy Example This chapter provides an overview of mass spectrometry, focusing on ionization sources and their significance in the development of mass. C 5 h 12 has an exact mass of 72.0939 amu, whereas c 4 h 8 o has an exact mass of 72.0575 amu. Mass spectrometry allows us to measure the masses of atoms and molecules, and also obtain information. Mass Spectroscopy Example.
From www.slideserve.com
PPT Mass Spectrometry PowerPoint Presentation, free download ID6357895 Mass Spectroscopy Example The three essential functions of a mass spectrometer, and the associated components, are: This chapter provides an overview of mass spectrometry, focusing on ionization sources and their significance in the development of mass. When molecules are bombarded with an energetic electron beam,. Mass spectroscopy is the accurate method for determining of molecular mass of the compound and its elemental composition.. Mass Spectroscopy Example.
From mungfali.com
Mass Spectrometry Principle Mass Spectroscopy Example A small sample is ionized, usually to cations by loss of an electron. For example, both c 5 h 12 and c 4 h 8 o have mw = 72, but they differ slightly beyond the decimal point: Mass spectroscopy is the accurate method for determining of molecular mass of the compound and its elemental composition. This chapter provides an. Mass Spectroscopy Example.
From www.slideshare.net
INTERPRETATION OF MASS SPECTROSCOPY Mass Spectroscopy Example For example, both c 5 h 12 and c 4 h 8 o have mw = 72, but they differ slightly beyond the decimal point: A small sample is ionized, usually to cations by loss of an electron. The three essential functions of a mass spectrometer, and the associated components, are: C 5 h 12 has an exact mass of. Mass Spectroscopy Example.
From chem.libretexts.org
4.3 Mass Spectrometry Chemistry LibreTexts Mass Spectroscopy Example Mass spectrometry allows us to measure the masses of atoms and molecules, and also obtain information about their chemical structure. If something is moving and you subject it to a sideways force, instead of moving in a straight. When molecules are bombarded with an energetic electron beam,. Mass spectroscopy is the accurate method for determining of molecular mass of the. Mass Spectroscopy Example.
From www.slideserve.com
PPT Mass Spectrometry Part 1 PowerPoint Presentation, free download Mass Spectroscopy Example If something is moving and you subject it to a sideways force, instead of moving in a straight. A small sample is ionized, usually to cations by loss of an electron. Before we talk about interpreting spectra, let’s. C 5 h 12 has an exact mass of 72.0939 amu, whereas c 4 h 8 o has an exact mass of. Mass Spectroscopy Example.
From hubpages.com
Mass Spectrometry HubPages Mass Spectroscopy Example Before we talk about interpreting spectra, let’s. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that they. The three essential functions of a mass spectrometer, and the associated components, are: For example, both c 5 h 12 and c 4 h 8 o have mw = 72, but they differ slightly. Mass Spectroscopy Example.
From study.com
How to Identify an Element from Its Mass Spectrum Chemistry Mass Spectroscopy Example A small sample is ionized, usually to cations by loss of an electron. Mass spectrometry allows us to measure the masses of atoms and molecules, and also obtain information about their chemical structure. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that they. This page describes how a mass spectrum is. Mass Spectroscopy Example.
From mavink.com
Icp Ms Spectrum Mass Spectroscopy Example C 5 h 12 has an exact mass of 72.0939 amu, whereas c 4 h 8 o has an exact mass of 72.0575 amu. A small sample is ionized, usually to cations by loss of an electron. Mass spectrometry allows us to measure the masses of atoms and molecules, and also obtain information about their chemical structure. If something is. Mass Spectroscopy Example.
From www.youtube.com
Mass Spectrometry Nitrogen Rule & Examples YouTube Mass Spectroscopy Example Before we talk about interpreting spectra, let’s. When molecules are bombarded with an energetic electron beam,. Mass spectroscopy is the accurate method for determining of molecular mass of the compound and its elemental composition. This chapter provides an overview of mass spectrometry, focusing on ionization sources and their significance in the development of mass. This page describes how a mass. Mass Spectroscopy Example.
From www.sliderbase.com
Mass Spectrometry Presentation Chemistry Mass Spectroscopy Example Before we talk about interpreting spectra, let’s. This chapter provides an overview of mass spectrometry, focusing on ionization sources and their significance in the development of mass. When molecules are bombarded with an energetic electron beam,. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that they. Mass spectroscopy is the accurate. Mass Spectroscopy Example.
From www.researchgate.net
Experimental approach of IRIS, combining mass spectrometry with Mass Spectroscopy Example The three essential functions of a mass spectrometer, and the associated components, are: A small sample is ionized, usually to cations by loss of an electron. This chapter provides an overview of mass spectrometry, focusing on ionization sources and their significance in the development of mass. If something is moving and you subject it to a sideways force, instead of. Mass Spectroscopy Example.
From www.researchgate.net
Mass spectrometry data showing the stability of the compounds. ac Mass Spectroscopy Example The three essential functions of a mass spectrometer, and the associated components, are: Mass spectrometry allows us to measure the masses of atoms and molecules, and also obtain information about their chemical structure. If something is moving and you subject it to a sideways force, instead of moving in a straight. For example, both c 5 h 12 and c. Mass Spectroscopy Example.
From biologydictionary.net
Mass Spectrometry The Definitive Guide Biology Dictionary Mass Spectroscopy Example This chapter provides an overview of mass spectrometry, focusing on ionization sources and their significance in the development of mass. If something is moving and you subject it to a sideways force, instead of moving in a straight. Mass spectrometry allows us to measure the masses of atoms and molecules, and also obtain information about their chemical structure. The three. Mass Spectroscopy Example.
From www.researchgate.net
Gas chromatography/mass spectrometry (GC/MS) chromatograms (full scan Mass Spectroscopy Example Before we talk about interpreting spectra, let’s. When molecules are bombarded with an energetic electron beam,. This page describes how a mass spectrum is produced using a mass spectrometer. For example, both c 5 h 12 and c 4 h 8 o have mw = 72, but they differ slightly beyond the decimal point: A small sample is ionized, usually. Mass Spectroscopy Example.
From socratic.org
What is the mass spectrum? + Example Mass Spectroscopy Example In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that they. Before we talk about interpreting spectra, let’s. A small sample is ionized, usually to cations by loss of an electron. C 5 h 12 has an exact mass of 72.0939 amu, whereas c 4 h 8 o has an exact mass. Mass Spectroscopy Example.
From www.compoundchem.com
Mass Spectrometry and Interpreting Mass Spectra Compound Interest Mass Spectroscopy Example Before we talk about interpreting spectra, let’s. Mass spectroscopy is the accurate method for determining of molecular mass of the compound and its elemental composition. The three essential functions of a mass spectrometer, and the associated components, are: When molecules are bombarded with an energetic electron beam,. A small sample is ionized, usually to cations by loss of an electron.. Mass Spectroscopy Example.
From www.youtube.com
Mass Spectroscopy The Difference Between M+ (Parent) Peak vs Base Peak Mass Spectroscopy Example Mass spectroscopy is the accurate method for determining of molecular mass of the compound and its elemental composition. A small sample is ionized, usually to cations by loss of an electron. When molecules are bombarded with an energetic electron beam,. This chapter provides an overview of mass spectrometry, focusing on ionization sources and their significance in the development of mass.. Mass Spectroscopy Example.
From www.instantuition.com
Mass Spectrometry of Chlorine O Level Chemistry Mass Spectroscopy Example When molecules are bombarded with an energetic electron beam,. This page describes how a mass spectrum is produced using a mass spectrometer. This chapter provides an overview of mass spectrometry, focusing on ionization sources and their significance in the development of mass. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that. Mass Spectroscopy Example.
From chem.libretexts.org
4.3 Mass Spectrometry Chemistry LibreTexts Mass Spectroscopy Example This page describes how a mass spectrum is produced using a mass spectrometer. The three essential functions of a mass spectrometer, and the associated components, are: C 5 h 12 has an exact mass of 72.0939 amu, whereas c 4 h 8 o has an exact mass of 72.0575 amu. Mass spectroscopy is the accurate method for determining of molecular. Mass Spectroscopy Example.
From www.slideserve.com
PPT Mass Spectrometry PowerPoint Presentation, free download ID2090241 Mass Spectroscopy Example This chapter provides an overview of mass spectrometry, focusing on ionization sources and their significance in the development of mass. If something is moving and you subject it to a sideways force, instead of moving in a straight. For example, both c 5 h 12 and c 4 h 8 o have mw = 72, but they differ slightly beyond. Mass Spectroscopy Example.
From www.slideshare.net
Mass spectrometry Mass Spectroscopy Example Mass spectroscopy is the accurate method for determining of molecular mass of the compound and its elemental composition. For example, both c 5 h 12 and c 4 h 8 o have mw = 72, but they differ slightly beyond the decimal point: In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so. Mass Spectroscopy Example.
From covalentmetrology.com
Gas Chromatography Mass Spectroscopy (GCMS) Material Testing Labs Mass Spectroscopy Example A small sample is ionized, usually to cations by loss of an electron. Before we talk about interpreting spectra, let’s. This page describes how a mass spectrum is produced using a mass spectrometer. This chapter provides an overview of mass spectrometry, focusing on ionization sources and their significance in the development of mass. Mass spectrometry allows us to measure the. Mass Spectroscopy Example.
From www.zimmermann.chemie.uni-rostock.de
High resolution mass spectrometry Analytische Chemie Universität Mass Spectroscopy Example This page describes how a mass spectrum is produced using a mass spectrometer. Mass spectrometry allows us to measure the masses of atoms and molecules, and also obtain information about their chemical structure. Before we talk about interpreting spectra, let’s. A small sample is ionized, usually to cations by loss of an electron. The three essential functions of a mass. Mass Spectroscopy Example.
From www.researchgate.net
Example of tandem mass spectrometry (MS/MS) spectrum of an isobaric tag Mass Spectroscopy Example Mass spectroscopy is the accurate method for determining of molecular mass of the compound and its elemental composition. C 5 h 12 has an exact mass of 72.0939 amu, whereas c 4 h 8 o has an exact mass of 72.0575 amu. When molecules are bombarded with an energetic electron beam,. If something is moving and you subject it to. Mass Spectroscopy Example.
From cexwkakx.blob.core.windows.net
Difference Between Mass Spectrometry And Mass Spectrophotometry at Emma Mass Spectroscopy Example When molecules are bombarded with an energetic electron beam,. C 5 h 12 has an exact mass of 72.0939 amu, whereas c 4 h 8 o has an exact mass of 72.0575 amu. This page describes how a mass spectrum is produced using a mass spectrometer. For example, both c 5 h 12 and c 4 h 8 o have. Mass Spectroscopy Example.
From www.researchgate.net
Examples of mass spectrometry fingerprints used for the identification Mass Spectroscopy Example Before we talk about interpreting spectra, let’s. Mass spectrometry allows us to measure the masses of atoms and molecules, and also obtain information about their chemical structure. A small sample is ionized, usually to cations by loss of an electron. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that they. This. Mass Spectroscopy Example.
From www.slideserve.com
PPT Biomarker Detection Using Mass Spectroscopy PowerPoint Mass Spectroscopy Example For example, both c 5 h 12 and c 4 h 8 o have mw = 72, but they differ slightly beyond the decimal point: In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that they. If something is moving and you subject it to a sideways force, instead of moving in. Mass Spectroscopy Example.
From www.slideserve.com
PPT Proteomics & Mass Spectrometry PowerPoint Presentation, free Mass Spectroscopy Example When molecules are bombarded with an energetic electron beam,. This page describes how a mass spectrum is produced using a mass spectrometer. If something is moving and you subject it to a sideways force, instead of moving in a straight. A small sample is ionized, usually to cations by loss of an electron. In order to measure the characteristics of. Mass Spectroscopy Example.
From www.jeolusa.com
Mass Spectrometry Basics Mass Spectrometry JEOL USA Mass Spectroscopy Example Before we talk about interpreting spectra, let’s. This chapter provides an overview of mass spectrometry, focusing on ionization sources and their significance in the development of mass. C 5 h 12 has an exact mass of 72.0939 amu, whereas c 4 h 8 o has an exact mass of 72.0575 amu. Mass spectroscopy is the accurate method for determining of. Mass Spectroscopy Example.
From www.chemistrystudent.com
Mass Spectrometry (ALevel) ChemistryStudent Mass Spectroscopy Example Before we talk about interpreting spectra, let’s. A small sample is ionized, usually to cations by loss of an electron. For example, both c 5 h 12 and c 4 h 8 o have mw = 72, but they differ slightly beyond the decimal point: The three essential functions of a mass spectrometer, and the associated components, are: In order. Mass Spectroscopy Example.
From www.youtube.com
Mass spectrometryMass spectroscopyMass spectrometerPrinciple Mass Spectroscopy Example Mass spectroscopy is the accurate method for determining of molecular mass of the compound and its elemental composition. A small sample is ionized, usually to cations by loss of an electron. This page describes how a mass spectrum is produced using a mass spectrometer. Before we talk about interpreting spectra, let’s. This chapter provides an overview of mass spectrometry, focusing. Mass Spectroscopy Example.
From chem.libretexts.org
4.3 Mass Spectrometry Chemistry LibreTexts Mass Spectroscopy Example The three essential functions of a mass spectrometer, and the associated components, are: This chapter provides an overview of mass spectrometry, focusing on ionization sources and their significance in the development of mass. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that they. C 5 h 12 has an exact mass. Mass Spectroscopy Example.
From chem.libretexts.org
4.3 Mass Spectrometry Chemistry LibreTexts Mass Spectroscopy Example Mass spectroscopy is the accurate method for determining of molecular mass of the compound and its elemental composition. When molecules are bombarded with an energetic electron beam,. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that they. C 5 h 12 has an exact mass of 72.0939 amu, whereas c 4. Mass Spectroscopy Example.
From www.researchgate.net
An example of liquid chromatographymass spectrometry (LCMS) analysis Mass Spectroscopy Example If something is moving and you subject it to a sideways force, instead of moving in a straight. In order to measure the characteristics of individual molecules, a mass spectrometer converts them to ions so that they. When molecules are bombarded with an energetic electron beam,. This page describes how a mass spectrum is produced using a mass spectrometer. For. Mass Spectroscopy Example.