Titration Lab Write Up . write your introduction. For a titration, the introduction should include information about what you hope to find out and what substance or. Titration is the process of determining the concentration of an unknown solution using a solution of known. goal of lab 10: start preparing for emergencies in the chemical lab. what is a titration? A titration is an analytical procedure used to determine the accurate concentration of a sample by. Prepare primary standard solutions of potassium hydrogen. prepare the acid sample in flask #2 for titration as you did sample #1 in step f above. Then titrate the solution with the. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution.
from modernalternativemama.com
prepare the acid sample in flask #2 for titration as you did sample #1 in step f above. write your introduction. For a titration, the introduction should include information about what you hope to find out and what substance or. goal of lab 10: what is a titration? Titration is the process of determining the concentration of an unknown solution using a solution of known. A titration is an analytical procedure used to determine the accurate concentration of a sample by. Then titrate the solution with the. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. start preparing for emergencies in the chemical lab.
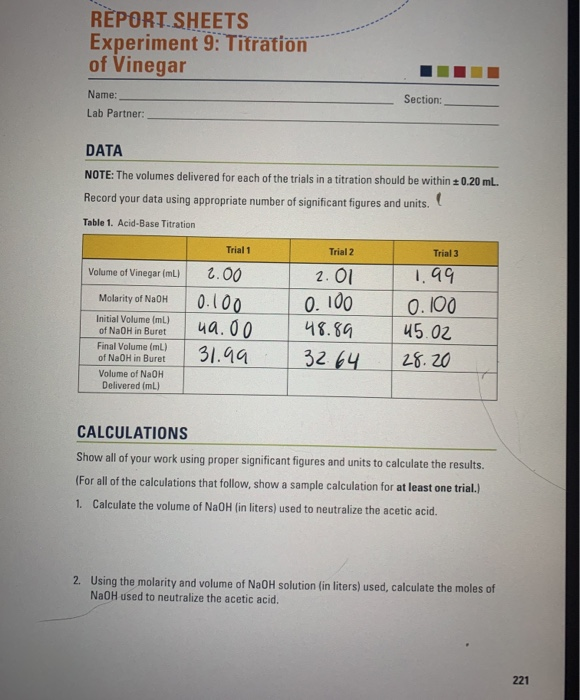
lab report titration
Titration Lab Write Up Prepare primary standard solutions of potassium hydrogen. For a titration, the introduction should include information about what you hope to find out and what substance or. prepare the acid sample in flask #2 for titration as you did sample #1 in step f above. what is a titration? start preparing for emergencies in the chemical lab. Titration is the process of determining the concentration of an unknown solution using a solution of known. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. write your introduction. A titration is an analytical procedure used to determine the accurate concentration of a sample by. goal of lab 10: Then titrate the solution with the. Prepare primary standard solutions of potassium hydrogen.
From www.thinkswap.com
Titration Practical Report Chemistry Year 11 SACE Thinkswap Titration Lab Write Up Then titrate the solution with the. start preparing for emergencies in the chemical lab. prepare the acid sample in flask #2 for titration as you did sample #1 in step f above. Prepare primary standard solutions of potassium hydrogen. For a titration, the introduction should include information about what you hope to find out and what substance or.. Titration Lab Write Up.
From www.thinkswap.com
Complete Acid/Base Titration Report Chemistry Year 12 HSC Thinkswap Titration Lab Write Up Then titrate the solution with the. goal of lab 10: in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. start preparing for emergencies in the chemical lab. what is a titration? Prepare primary standard solutions of potassium hydrogen. For a titration, the introduction should include information. Titration Lab Write Up.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration Lab Write Up For a titration, the introduction should include information about what you hope to find out and what substance or. start preparing for emergencies in the chemical lab. Prepare primary standard solutions of potassium hydrogen. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Titration is the process of. Titration Lab Write Up.
From ebinu.blog
AcidBase Titration Lab — DataClassroom / Acid Base Titration Titration Lab Write Up start preparing for emergencies in the chemical lab. write your introduction. Prepare primary standard solutions of potassium hydrogen. Titration is the process of determining the concentration of an unknown solution using a solution of known. goal of lab 10: Then titrate the solution with the. For a titration, the introduction should include information about what you hope. Titration Lab Write Up.
From www.scribd.com
titration ( chemistry Experiment report) Titration Analysis Titration Lab Write Up start preparing for emergencies in the chemical lab. Then titrate the solution with the. prepare the acid sample in flask #2 for titration as you did sample #1 in step f above. Titration is the process of determining the concentration of an unknown solution using a solution of known. goal of lab 10: write your introduction.. Titration Lab Write Up.
From www.markedbyteachers.com
Assessed Practical Titration Writeup ALevel Science Marked by Titration Lab Write Up in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Then titrate the solution with the. A titration is an analytical procedure used to determine the accurate concentration of a sample by. goal of lab 10: start preparing for emergencies in the chemical lab. what is a. Titration Lab Write Up.
From www.sliderbase.com
Titration Titration Lab Write Up A titration is an analytical procedure used to determine the accurate concentration of a sample by. Titration is the process of determining the concentration of an unknown solution using a solution of known. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Prepare primary standard solutions of potassium hydrogen.. Titration Lab Write Up.
From olympiapublishers.com
Acid Base Titration Experiment Report Titration Lab Write Up write your introduction. prepare the acid sample in flask #2 for titration as you did sample #1 in step f above. A titration is an analytical procedure used to determine the accurate concentration of a sample by. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. . Titration Lab Write Up.
From www.studocu.com
Molar Mass of Citric Acid Using Titration Lab write up Riley Titration Lab Write Up goal of lab 10: A titration is an analytical procedure used to determine the accurate concentration of a sample by. write your introduction. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. start preparing for emergencies in the chemical lab. Then titrate the solution with the.. Titration Lab Write Up.
From studylib.net
Acid/Base Titration Lab Titration Lab Write Up what is a titration? Prepare primary standard solutions of potassium hydrogen. prepare the acid sample in flask #2 for titration as you did sample #1 in step f above. For a titration, the introduction should include information about what you hope to find out and what substance or. start preparing for emergencies in the chemical lab. Then. Titration Lab Write Up.
From www.markedbyteachers.com
Titration experiment write up GCSE Science Marked by Titration Lab Write Up start preparing for emergencies in the chemical lab. A titration is an analytical procedure used to determine the accurate concentration of a sample by. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. write your introduction. prepare the acid sample in flask #2 for titration as. Titration Lab Write Up.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration Lab Write Up goal of lab 10: in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Titration is the process of determining the concentration of an unknown solution using a solution of known. A titration is an analytical procedure used to determine the accurate concentration of a sample by. what. Titration Lab Write Up.
From www.studocu.com
Lecture Notes 9 + Experiment 9 ACIDBASE TITRATION Experiment 9 Titration Lab Write Up Titration is the process of determining the concentration of an unknown solution using a solution of known. Prepare primary standard solutions of potassium hydrogen. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. write your introduction. goal of lab 10: For a titration, the introduction should include. Titration Lab Write Up.
From caen-sccm-cdp01.engin.umich.edu
💄 Discussion acid base titration lab report. Acid Base Titration Titration Lab Write Up goal of lab 10: A titration is an analytical procedure used to determine the accurate concentration of a sample by. Then titrate the solution with the. start preparing for emergencies in the chemical lab. For a titration, the introduction should include information about what you hope to find out and what substance or. Titration is the process of. Titration Lab Write Up.
From slidesharetrick.blogspot.com
Acid Base Titration Lab Report slidesharetrick Titration Lab Write Up in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. A titration is an analytical procedure used to determine the accurate concentration of a sample by. prepare the acid sample in flask #2 for titration as you did sample #1 in step f above. Then titrate the solution with. Titration Lab Write Up.
From general.chemistrysteps.com
Strong AcidStrong Base Titrations Chemistry Steps Titration Lab Write Up A titration is an analytical procedure used to determine the accurate concentration of a sample by. start preparing for emergencies in the chemical lab. For a titration, the introduction should include information about what you hope to find out and what substance or. goal of lab 10: Then titrate the solution with the. Titration is the process of. Titration Lab Write Up.
From www.olivier-martel.com
Example Lab Report Chemistry Titration Example Lab Report Chemistry Titration Lab Write Up goal of lab 10: in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. start preparing for emergencies in the chemical lab. Titration is the process of determining the concentration of an unknown solution using a solution of known. Prepare primary standard solutions of potassium hydrogen. write. Titration Lab Write Up.
From studylib.net
CHEMISTRY I LAB INTRO TO TITRATION Titration Lab Write Up A titration is an analytical procedure used to determine the accurate concentration of a sample by. Then titrate the solution with the. goal of lab 10: start preparing for emergencies in the chemical lab. Titration is the process of determining the concentration of an unknown solution using a solution of known. what is a titration? prepare. Titration Lab Write Up.
From www.scribd.com
Lab Report Sample[1] Titration Chemistry Titration Lab Write Up in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. For a titration, the introduction should include information about what you hope to find out and what substance or. what is a titration? Prepare primary standard solutions of potassium hydrogen. start preparing for emergencies in the chemical lab.. Titration Lab Write Up.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Titration Lab Write Up For a titration, the introduction should include information about what you hope to find out and what substance or. prepare the acid sample in flask #2 for titration as you did sample #1 in step f above. Prepare primary standard solutions of potassium hydrogen. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble. Titration Lab Write Up.
From www.science-revision.co.uk
Titrations Titration Lab Write Up Titration is the process of determining the concentration of an unknown solution using a solution of known. For a titration, the introduction should include information about what you hope to find out and what substance or. A titration is an analytical procedure used to determine the accurate concentration of a sample by. goal of lab 10: Prepare primary standard. Titration Lab Write Up.
From www.docsity.com
Acid Base Titrations Lab Report General Chemistry Lab CHEM 1045 Titration Lab Write Up Prepare primary standard solutions of potassium hydrogen. what is a titration? prepare the acid sample in flask #2 for titration as you did sample #1 in step f above. A titration is an analytical procedure used to determine the accurate concentration of a sample by. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce. Titration Lab Write Up.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration Lab Write Up For a titration, the introduction should include information about what you hope to find out and what substance or. start preparing for emergencies in the chemical lab. what is a titration? Prepare primary standard solutions of potassium hydrogen. A titration is an analytical procedure used to determine the accurate concentration of a sample by. prepare the acid. Titration Lab Write Up.
From www.slideshare.net
Titration Lab Report Titration Lab Write Up For a titration, the introduction should include information about what you hope to find out and what substance or. in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. start preparing for emergencies in the chemical lab. Prepare primary standard solutions of potassium hydrogen. prepare the acid sample. Titration Lab Write Up.
From letitsnowglobe.co.uk
Titration procedure pdf Titration Lab Write Up prepare the acid sample in flask #2 for titration as you did sample #1 in step f above. Titration is the process of determining the concentration of an unknown solution using a solution of known. Prepare primary standard solutions of potassium hydrogen. goal of lab 10: Then titrate the solution with the. start preparing for emergencies in. Titration Lab Write Up.
From studylib.net
Titration Lab Report Walker Singleton Titration Lab Write Up what is a titration? Titration is the process of determining the concentration of an unknown solution using a solution of known. A titration is an analytical procedure used to determine the accurate concentration of a sample by. start preparing for emergencies in the chemical lab. Then titrate the solution with the. goal of lab 10: Prepare primary. Titration Lab Write Up.
From www.slideshare.net
Chemistry Titration Lab Report Handout Titration Lab Write Up start preparing for emergencies in the chemical lab. For a titration, the introduction should include information about what you hope to find out and what substance or. A titration is an analytical procedure used to determine the accurate concentration of a sample by. Prepare primary standard solutions of potassium hydrogen. in this experiment students neutralise sodium hydroxide with. Titration Lab Write Up.
From www.slideshare.net
Titration experiment report Titration Lab Write Up Then titrate the solution with the. Prepare primary standard solutions of potassium hydrogen. Titration is the process of determining the concentration of an unknown solution using a solution of known. write your introduction. start preparing for emergencies in the chemical lab. prepare the acid sample in flask #2 for titration as you did sample #1 in step. Titration Lab Write Up.
From studylib.net
Titration Lab Titration Lab Write Up prepare the acid sample in flask #2 for titration as you did sample #1 in step f above. what is a titration? Titration is the process of determining the concentration of an unknown solution using a solution of known. For a titration, the introduction should include information about what you hope to find out and what substance or.. Titration Lab Write Up.
From www.scribd.com
LAB Report 3 Titration Chemistry Titration Lab Write Up in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the soluble salt sodium chloride in solution. Prepare primary standard solutions of potassium hydrogen. A titration is an analytical procedure used to determine the accurate concentration of a sample by. write your introduction. For a titration, the introduction should include information about what you hope to. Titration Lab Write Up.
From www.studocu.com
Titration Lab Report About the lab tiration Titration Lab Report Titration Lab Write Up For a titration, the introduction should include information about what you hope to find out and what substance or. Then titrate the solution with the. start preparing for emergencies in the chemical lab. Prepare primary standard solutions of potassium hydrogen. what is a titration? in this experiment students neutralise sodium hydroxide with hydrochloric acid to produce the. Titration Lab Write Up.
From yamiletsapchemistrylabs.weebly.com
Titration Lab Yamilet's AP Chemistry Labs Titration Lab Write Up write your introduction. goal of lab 10: prepare the acid sample in flask #2 for titration as you did sample #1 in step f above. Titration is the process of determining the concentration of an unknown solution using a solution of known. Prepare primary standard solutions of potassium hydrogen. A titration is an analytical procedure used to. Titration Lab Write Up.
From modernalternativemama.com
lab report titration Titration Lab Write Up Then titrate the solution with the. what is a titration? A titration is an analytical procedure used to determine the accurate concentration of a sample by. For a titration, the introduction should include information about what you hope to find out and what substance or. Titration is the process of determining the concentration of an unknown solution using a. Titration Lab Write Up.
From parkermcyrandolph.blogspot.com
Acid Base Titration Lab Report ParkermcyRandolph Titration Lab Write Up start preparing for emergencies in the chemical lab. Titration is the process of determining the concentration of an unknown solution using a solution of known. write your introduction. goal of lab 10: A titration is an analytical procedure used to determine the accurate concentration of a sample by. For a titration, the introduction should include information about. Titration Lab Write Up.
From studylib.net
Lab Titration Titration Lab Write Up prepare the acid sample in flask #2 for titration as you did sample #1 in step f above. A titration is an analytical procedure used to determine the accurate concentration of a sample by. For a titration, the introduction should include information about what you hope to find out and what substance or. Titration is the process of determining. Titration Lab Write Up.