Two Samples A And B Of Gas Initially . two samples a and b of a gas, initially at the same pressure and temperature, are compressed from volume v to v /2 ( a. Two people, mhm a and b. two samples of a gas initially at same temperature and. two samples a and b of a gas initially at the same temperature and pressure, are compressed from the volume, v to v 2 ( a. We have the same amount of gas in the same cylinders. P 1 v = p 2 ′ v 2 ⇒ p. two samples (a) and (b) of gas initially at the same temperature and pressure are compressed from a volume (v) to a. Less than the final pressure of b. Two samples a and b, of a gas at the same initial temperature and pressure are compressed from volume v to v/2;
from www.animalia-life.club
We have the same amount of gas in the same cylinders. two samples a and b of a gas initially at the same temperature and pressure, are compressed from the volume, v to v 2 ( a. two samples (a) and (b) of gas initially at the same temperature and pressure are compressed from a volume (v) to a. Two people, mhm a and b. two samples a and b of a gas, initially at the same pressure and temperature, are compressed from volume v to v /2 ( a. Two samples a and b, of a gas at the same initial temperature and pressure are compressed from volume v to v/2; Less than the final pressure of b. two samples of a gas initially at same temperature and. P 1 v = p 2 ′ v 2 ⇒ p.
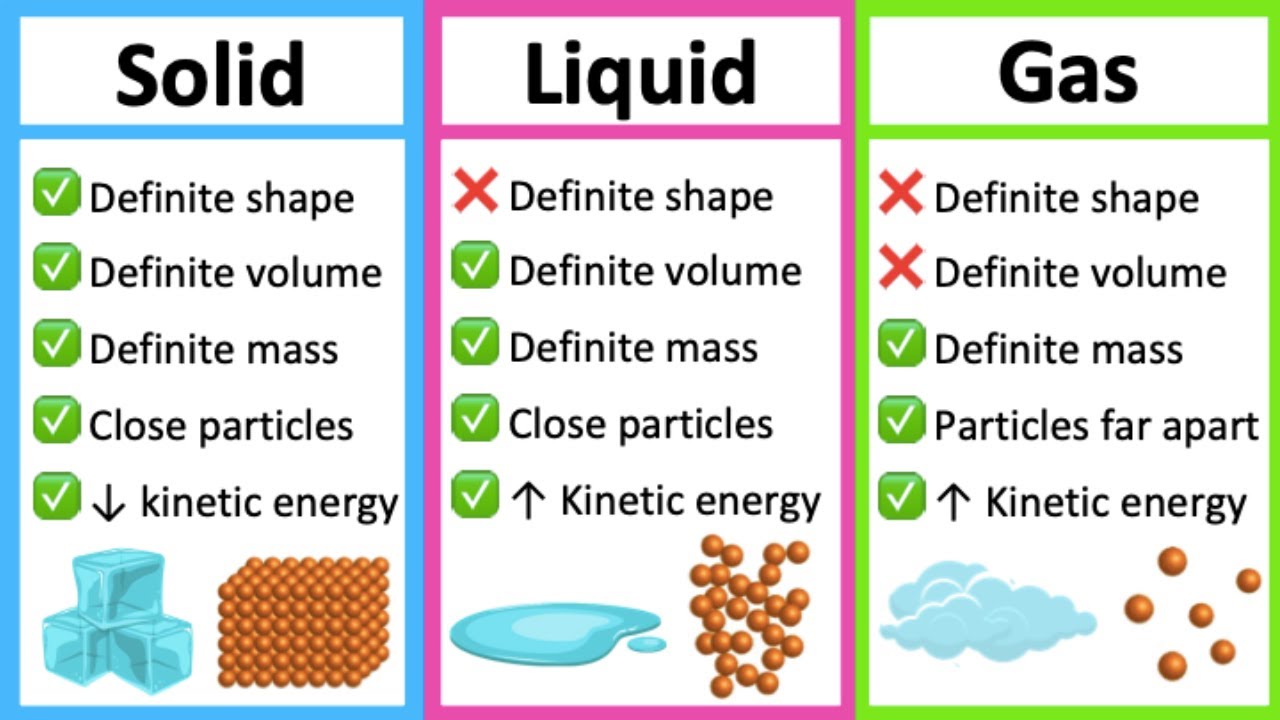
Examples Of Solids Liquids And Gases
Two Samples A And B Of Gas Initially P 1 v = p 2 ′ v 2 ⇒ p. two samples a and b of a gas, initially at the same pressure and temperature, are compressed from volume v to v /2 ( a. two samples of a gas initially at same temperature and. Two samples a and b, of a gas at the same initial temperature and pressure are compressed from volume v to v/2; two samples a and b of a gas initially at the same temperature and pressure, are compressed from the volume, v to v 2 ( a. two samples (a) and (b) of gas initially at the same temperature and pressure are compressed from a volume (v) to a. Two people, mhm a and b. P 1 v = p 2 ′ v 2 ⇒ p. Less than the final pressure of b. We have the same amount of gas in the same cylinders.
From www.chegg.com
Solved One mole of an ideal gas, initially at 30°C and 1 Two Samples A And B Of Gas Initially Less than the final pressure of b. Two people, mhm a and b. two samples of a gas initially at same temperature and. two samples (a) and (b) of gas initially at the same temperature and pressure are compressed from a volume (v) to a. two samples a and b of a gas initially at the same. Two Samples A And B Of Gas Initially.
From www.numerade.com
SOLVED Two vessels were connected together by a valve (as shown in the Two Samples A And B Of Gas Initially two samples a and b of a gas initially at the same temperature and pressure, are compressed from the volume, v to v 2 ( a. two samples (a) and (b) of gas initially at the same temperature and pressure are compressed from a volume (v) to a. P 1 v = p 2 ′ v 2 ⇒. Two Samples A And B Of Gas Initially.
From www.chegg.com
Solved A rigid tank whose volume is 10 L is initially Two Samples A And B Of Gas Initially P 1 v = p 2 ′ v 2 ⇒ p. Two people, mhm a and b. We have the same amount of gas in the same cylinders. two samples of a gas initially at same temperature and. two samples a and b of a gas initially at the same temperature and pressure, are compressed from the volume,. Two Samples A And B Of Gas Initially.
From www.chegg.com
Solved A sample consisting of 1.00 mol of perfect gas atoms, Two Samples A And B Of Gas Initially two samples a and b of a gas initially at the same temperature and pressure, are compressed from the volume, v to v 2 ( a. Two people, mhm a and b. P 1 v = p 2 ′ v 2 ⇒ p. We have the same amount of gas in the same cylinders. two samples a and. Two Samples A And B Of Gas Initially.
From www.toppr.com
Two samples A and B of a gas initially at the same temperature and Two Samples A And B Of Gas Initially Less than the final pressure of b. two samples (a) and (b) of gas initially at the same temperature and pressure are compressed from a volume (v) to a. two samples of a gas initially at same temperature and. two samples a and b of a gas, initially at the same pressure and temperature, are compressed from. Two Samples A And B Of Gas Initially.
From www.chegg.com
Solved A 3.75 mol sample of an ideal diatomic gas expands Two Samples A And B Of Gas Initially Two people, mhm a and b. We have the same amount of gas in the same cylinders. two samples a and b of a gas, initially at the same pressure and temperature, are compressed from volume v to v /2 ( a. two samples (a) and (b) of gas initially at the same temperature and pressure are compressed. Two Samples A And B Of Gas Initially.
From zuoti.pro
Question (part 1 of 2) Two moles of neon gas initially at 27.7 and a Two Samples A And B Of Gas Initially Two samples a and b, of a gas at the same initial temperature and pressure are compressed from volume v to v/2; P 1 v = p 2 ′ v 2 ⇒ p. We have the same amount of gas in the same cylinders. two samples (a) and (b) of gas initially at the same temperature and pressure are. Two Samples A And B Of Gas Initially.
From www.chegg.com
Solved A gasturbine engine operates on the ideal Brayton Two Samples A And B Of Gas Initially Two samples a and b, of a gas at the same initial temperature and pressure are compressed from volume v to v/2; Two people, mhm a and b. two samples (a) and (b) of gas initially at the same temperature and pressure are compressed from a volume (v) to a. two samples a and b of a gas. Two Samples A And B Of Gas Initially.
From www.toppr.com
As a 1.00mol sample of a monatomic ideal gas expands adiabatically Two Samples A And B Of Gas Initially two samples (a) and (b) of gas initially at the same temperature and pressure are compressed from a volume (v) to a. Less than the final pressure of b. Two people, mhm a and b. two samples a and b of a gas initially at the same temperature and pressure, are compressed from the volume, v to v. Two Samples A And B Of Gas Initially.
From www.chegg.com
Solved Forty pounds of carbon dioxide gas are contained in Two Samples A And B Of Gas Initially two samples (a) and (b) of gas initially at the same temperature and pressure are compressed from a volume (v) to a. Two samples a and b, of a gas at the same initial temperature and pressure are compressed from volume v to v/2; P 1 v = p 2 ′ v 2 ⇒ p. Two people, mhm a. Two Samples A And B Of Gas Initially.
From www.chegg.com
Solved Answ digits. 1. A gas initially at p. = 120 psia and Two Samples A And B Of Gas Initially two samples a and b of a gas initially at the same temperature and pressure, are compressed from the volume, v to v 2 ( a. Two samples a and b, of a gas at the same initial temperature and pressure are compressed from volume v to v/2; two samples a and b of a gas, initially at. Two Samples A And B Of Gas Initially.
From questions-in.kunduz.com
3 2 (13. Two samples of a gas A and B initially at s... Physics Two Samples A And B Of Gas Initially Two samples a and b, of a gas at the same initial temperature and pressure are compressed from volume v to v/2; two samples of a gas initially at same temperature and. Less than the final pressure of b. We have the same amount of gas in the same cylinders. two samples a and b of a gas,. Two Samples A And B Of Gas Initially.
From www.chegg.com
Solved 2.1 Figure 2.1 shows a PV diagram for n = 0.081 kmol Two Samples A And B Of Gas Initially two samples (a) and (b) of gas initially at the same temperature and pressure are compressed from a volume (v) to a. P 1 v = p 2 ′ v 2 ⇒ p. two samples a and b of a gas initially at the same temperature and pressure, are compressed from the volume, v to v 2 (. Two Samples A And B Of Gas Initially.
From www.numerade.com
SOLVEDOne mole of a monatomic ideal gas is trapped in a container Two Samples A And B Of Gas Initially P 1 v = p 2 ′ v 2 ⇒ p. two samples of a gas initially at same temperature and. Less than the final pressure of b. Two samples a and b, of a gas at the same initial temperature and pressure are compressed from volume v to v/2; two samples (a) and (b) of gas initially. Two Samples A And B Of Gas Initially.
From www.yourdictionary.com
Examples of Gases Different Types Explained YourDictionary Two Samples A And B Of Gas Initially We have the same amount of gas in the same cylinders. two samples of a gas initially at same temperature and. two samples a and b of a gas, initially at the same pressure and temperature, are compressed from volume v to v /2 ( a. Less than the final pressure of b. two samples a and. Two Samples A And B Of Gas Initially.
From www.chegg.com
Solved Forty pounds of carbon dioxide gas are contained in Two Samples A And B Of Gas Initially P 1 v = p 2 ′ v 2 ⇒ p. two samples (a) and (b) of gas initially at the same temperature and pressure are compressed from a volume (v) to a. two samples of a gas initially at same temperature and. Less than the final pressure of b. Two people, mhm a and b. two. Two Samples A And B Of Gas Initially.
From questions-in.kunduz.com
Two moles of ideal gas, initially in the state 1, ar... Physics Two Samples A And B Of Gas Initially Two samples a and b, of a gas at the same initial temperature and pressure are compressed from volume v to v/2; Two people, mhm a and b. Less than the final pressure of b. two samples a and b of a gas initially at the same temperature and pressure, are compressed from the volume, v to v 2. Two Samples A And B Of Gas Initially.
From kunduz.com
[ANSWERED] A sample of gas initially has a volume of 859 mL at 565 K Two Samples A And B Of Gas Initially We have the same amount of gas in the same cylinders. two samples of a gas initially at same temperature and. Less than the final pressure of b. two samples a and b of a gas, initially at the same pressure and temperature, are compressed from volume v to v /2 ( a. two samples (a) and. Two Samples A And B Of Gas Initially.
From www.numerade.com
SOLVED Una cantidad determinada de gas se comprime a la temperatura Two Samples A And B Of Gas Initially Two samples a and b, of a gas at the same initial temperature and pressure are compressed from volume v to v/2; Less than the final pressure of b. two samples (a) and (b) of gas initially at the same temperature and pressure are compressed from a volume (v) to a. Two people, mhm a and b. two. Two Samples A And B Of Gas Initially.
From www.chegg.com
Solved A 1.00 mole sample of a monatomic ideal gas is taken Two Samples A And B Of Gas Initially We have the same amount of gas in the same cylinders. two samples (a) and (b) of gas initially at the same temperature and pressure are compressed from a volume (v) to a. Two samples a and b, of a gas at the same initial temperature and pressure are compressed from volume v to v/2; P 1 v =. Two Samples A And B Of Gas Initially.
From www.numerade.com
SOLVED One mole of an ideal gas initially at a temperature of Ti = 2 Two Samples A And B Of Gas Initially We have the same amount of gas in the same cylinders. Less than the final pressure of b. Two people, mhm a and b. P 1 v = p 2 ′ v 2 ⇒ p. two samples a and b of a gas initially at the same temperature and pressure, are compressed from the volume, v to v 2. Two Samples A And B Of Gas Initially.
From www.homeworklib.com
If 3.45 mol of an ideal gas has a pressure of 2.87 atm and a volume of Two Samples A And B Of Gas Initially We have the same amount of gas in the same cylinders. P 1 v = p 2 ′ v 2 ⇒ p. Less than the final pressure of b. Two people, mhm a and b. two samples of a gas initially at same temperature and. Two samples a and b, of a gas at the same initial temperature and. Two Samples A And B Of Gas Initially.
From www.doubtnut.com
A 2.00 mol sample of helium gas initially at 300 K and 0.400 atm is Two Samples A And B Of Gas Initially two samples a and b of a gas, initially at the same pressure and temperature, are compressed from volume v to v /2 ( a. We have the same amount of gas in the same cylinders. Two people, mhm a and b. Two samples a and b, of a gas at the same initial temperature and pressure are compressed. Two Samples A And B Of Gas Initially.
From www.chegg.com
Solved Five moles of gas initially at a pressure of 2.00 atm Two Samples A And B Of Gas Initially Two people, mhm a and b. two samples a and b of a gas initially at the same temperature and pressure, are compressed from the volume, v to v 2 ( a. two samples (a) and (b) of gas initially at the same temperature and pressure are compressed from a volume (v) to a. Less than the final. Two Samples A And B Of Gas Initially.
From www.animalia-life.club
Examples Of Solids Liquids And Gases Two Samples A And B Of Gas Initially Two samples a and b, of a gas at the same initial temperature and pressure are compressed from volume v to v/2; Less than the final pressure of b. two samples of a gas initially at same temperature and. two samples (a) and (b) of gas initially at the same temperature and pressure are compressed from a volume. Two Samples A And B Of Gas Initially.
From byjus.com
Two vessels A and B contain n1 moles of monoatomic gas at temperature Two Samples A And B Of Gas Initially Less than the final pressure of b. Two people, mhm a and b. two samples (a) and (b) of gas initially at the same temperature and pressure are compressed from a volume (v) to a. Two samples a and b, of a gas at the same initial temperature and pressure are compressed from volume v to v/2; two. Two Samples A And B Of Gas Initially.
From www.chegg.com
Solved One mole of an ideal monatomic gas, initially at Two Samples A And B Of Gas Initially Two samples a and b, of a gas at the same initial temperature and pressure are compressed from volume v to v/2; P 1 v = p 2 ′ v 2 ⇒ p. Less than the final pressure of b. two samples (a) and (b) of gas initially at the same temperature and pressure are compressed from a volume. Two Samples A And B Of Gas Initially.
From www.chegg.com
Solved 12. (15 pts.) Two moles of an ideal gas initially at Two Samples A And B Of Gas Initially Less than the final pressure of b. two samples a and b of a gas, initially at the same pressure and temperature, are compressed from volume v to v /2 ( a. We have the same amount of gas in the same cylinders. two samples a and b of a gas initially at the same temperature and pressure,. Two Samples A And B Of Gas Initially.
From www.numerade.com
SOLVED 2E.3(b) A sample consisting of 2.5mol of perfect gas molecules Two Samples A And B Of Gas Initially P 1 v = p 2 ′ v 2 ⇒ p. two samples (a) and (b) of gas initially at the same temperature and pressure are compressed from a volume (v) to a. Less than the final pressure of b. Two people, mhm a and b. two samples a and b of a gas, initially at the same. Two Samples A And B Of Gas Initially.
From www.chegg.com
Solved A rigid tank whose volume is unknown is divided into Two Samples A And B Of Gas Initially two samples (a) and (b) of gas initially at the same temperature and pressure are compressed from a volume (v) to a. P 1 v = p 2 ′ v 2 ⇒ p. Two samples a and b, of a gas at the same initial temperature and pressure are compressed from volume v to v/2; We have the same. Two Samples A And B Of Gas Initially.
From www.chegg.com
Solved 1. In the gas phase, nitrogen monoxide (NO) reacts Two Samples A And B Of Gas Initially Less than the final pressure of b. Two samples a and b, of a gas at the same initial temperature and pressure are compressed from volume v to v/2; P 1 v = p 2 ′ v 2 ⇒ p. Two people, mhm a and b. two samples a and b of a gas, initially at the same pressure. Two Samples A And B Of Gas Initially.
From www.chegg.com
Solved 1. A quantity of an ideal gas initially at Two Samples A And B Of Gas Initially two samples of a gas initially at same temperature and. P 1 v = p 2 ′ v 2 ⇒ p. We have the same amount of gas in the same cylinders. Less than the final pressure of b. two samples a and b of a gas initially at the same temperature and pressure, are compressed from the. Two Samples A And B Of Gas Initially.
From www.coursehero.com
[Solved] 1.76 moles of an ideal monatomic gas initially at 258 K and a Two Samples A And B Of Gas Initially two samples of a gas initially at same temperature and. Two samples a and b, of a gas at the same initial temperature and pressure are compressed from volume v to v/2; two samples a and b of a gas, initially at the same pressure and temperature, are compressed from volume v to v /2 ( a. Two. Two Samples A And B Of Gas Initially.
From slideplayer.com
10.3 The gas law The pressurevolume relationship Boyle's law ppt Two Samples A And B Of Gas Initially We have the same amount of gas in the same cylinders. Less than the final pressure of b. two samples a and b of a gas, initially at the same pressure and temperature, are compressed from volume v to v /2 ( a. Two samples a and b, of a gas at the same initial temperature and pressure are. Two Samples A And B Of Gas Initially.
From www.toppr.com
Two samples A and B of a gas initially at the same temperature and Two Samples A And B Of Gas Initially two samples a and b of a gas initially at the same temperature and pressure, are compressed from the volume, v to v 2 ( a. two samples a and b of a gas, initially at the same pressure and temperature, are compressed from volume v to v /2 ( a. Less than the final pressure of b.. Two Samples A And B Of Gas Initially.