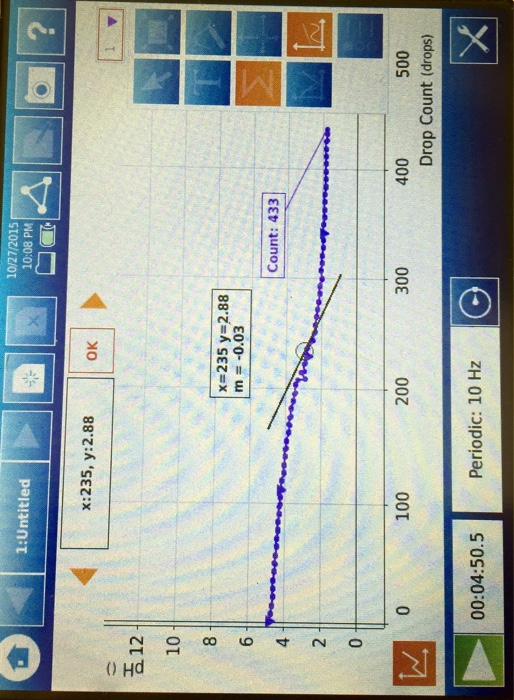
Difference Between Buffer And Titration . the relationship between titrations and buffers. a titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. There is a strong correlation between the effectiveness of a buffer. why are buffers important in our bodies and in nature? buffers are characterized by their ph range and buffer capacity. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. By this point, you are familiar with the concepts of acids and bases, and that.
from www.chegg.com
the relationship between titrations and buffers. why are buffers important in our bodies and in nature? A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. By this point, you are familiar with the concepts of acids and bases, and that. titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. There is a strong correlation between the effectiveness of a buffer. buffers are characterized by their ph range and buffer capacity. a titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant.
Solved Part 2 Titration of Acetate Buffer with HCl and NaOH
Difference Between Buffer And Titration a titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. a titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. buffers are characterized by their ph range and buffer capacity. the relationship between titrations and buffers. There is a strong correlation between the effectiveness of a buffer. By this point, you are familiar with the concepts of acids and bases, and that. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. why are buffers important in our bodies and in nature?
From pediaa.com
Difference Between AcidBase Titration and Redox Titration Difference Between Buffer And Titration A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. There is a strong correlation between the effectiveness of a buffer. the. Difference Between Buffer And Titration.
From brunofuga.adv.br
Difference Between AcidBase Titration And Redox Titration, 50 OFF Difference Between Buffer And Titration a titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. By this point, you are familiar with the. Difference Between Buffer And Titration.
From www.numerade.com
SOLVED what are the differences and similarities of titration weak Difference Between Buffer And Titration a titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. why are buffers important in our bodies and in nature? By this point, you are familiar with the concepts of acids and bases, and that. titration is a procedure used in chemistry in. Difference Between Buffer And Titration.
From chem.libretexts.org
17.3 AcidBase Titrations Chemistry LibreTexts Difference Between Buffer And Titration a titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. buffers are characterized by their ph range and buffer capacity. By this point, you are familiar with the concepts of acids and bases, and that. the relationship between titrations and buffers. A chemical. Difference Between Buffer And Titration.
From dokumen.tips
(PDF) Buffers and Titration DOKUMEN.TIPS Difference Between Buffer And Titration buffers are characterized by their ph range and buffer capacity. titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. There is a strong correlation between the effectiveness of a buffer. By this point, you are familiar with the concepts of acids and bases, and that. a titration. Difference Between Buffer And Titration.
From saylordotorg.github.io
Buffers Difference Between Buffer And Titration By this point, you are familiar with the concepts of acids and bases, and that. titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. There is a strong correlation between the effectiveness of a buffer. the relationship between titrations and buffers. buffers are characterized by their ph. Difference Between Buffer And Titration.
From www.youtube.com
Buffers and titration curve YouTube Difference Between Buffer And Titration A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. a titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. There is a strong correlation between the effectiveness. Difference Between Buffer And Titration.
From chemistry.stackexchange.com
Titration of CH3COONa with HCl and pKa determination from half Difference Between Buffer And Titration A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. There is a strong correlation between the effectiveness of a buffer. the relationship between titrations and buffers. why are buffers important in our bodies and in nature? titration is a. Difference Between Buffer And Titration.
From www.differencebetween.com
Difference Between Volumetric and Potentiometric Titration Compare Difference Between Buffer And Titration A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. a titration curve is a graph that relates the change in ph. Difference Between Buffer And Titration.
From slideplayer.com
Aqueous Equilibria Chapter ppt download Difference Between Buffer And Titration why are buffers important in our bodies and in nature? a titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. By this point, you are familiar with the concepts of acids and bases, and that. titration is a procedure used in chemistry in. Difference Between Buffer And Titration.
From www.youtube.com
Question 19. What are masking & demasking agent. Explain use of buffer Difference Between Buffer And Titration a titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. buffers are characterized by their ph range and buffer capacity. why are buffers important in our bodies and in nature? A chemical reaction is set up between a known volume of a solution. Difference Between Buffer And Titration.
From fyoizufqj.blob.core.windows.net
Equivalence Point Amino Acid Titration at Christine Grosso blog Difference Between Buffer And Titration titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. There is a strong correlation between the effectiveness of a buffer. buffers are characterized by their ph range and buffer capacity. By this point, you are familiar with the concepts of acids and bases, and that. A chemical reaction. Difference Between Buffer And Titration.
From fyospnfgs.blob.core.windows.net
Titration Curve How To Read at Elizabeth Moore blog Difference Between Buffer And Titration There is a strong correlation between the effectiveness of a buffer. the relationship between titrations and buffers. why are buffers important in our bodies and in nature? buffers are characterized by their ph range and buffer capacity. By this point, you are familiar with the concepts of acids and bases, and that. A chemical reaction is set. Difference Between Buffer And Titration.
From www.chegg.com
Solved Part 2 Titration of Acetate Buffer with HCl and NaOH Difference Between Buffer And Titration By this point, you are familiar with the concepts of acids and bases, and that. the relationship between titrations and buffers. a titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. titration is a procedure used in chemistry in order to determine the. Difference Between Buffer And Titration.
From thenoveldifference.com
Titration and Neutralization 6 Fancy Difference Difference Between Buffer And Titration a titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. the relationship between titrations and buffers. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. By. Difference Between Buffer And Titration.
From www.differencebetween.com
Difference Between Titration and Neutralization Compare the Difference Between Buffer And Titration the relationship between titrations and buffers. a titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. why are buffers important in our bodies and in nature? By this point, you are familiar with the concepts of acids and bases, and that. A chemical. Difference Between Buffer And Titration.
From unistudium.unipg.it
AcidBase Titration Curves Difference Between Buffer And Titration buffers are characterized by their ph range and buffer capacity. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. why are buffers important in our bodies and in nature? titration is a procedure used in chemistry in order to. Difference Between Buffer And Titration.
From ar.inspiredpencil.com
Titration Curve Buffer Region Difference Between Buffer And Titration titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. why are buffers important in our bodies and in nature? A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. By this. Difference Between Buffer And Titration.
From www.youtube.com
Mrs. KJ Explains 4.04 Buffers and Titration YouTube Difference Between Buffer And Titration A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. why are buffers important in our bodies and in nature? There is a strong correlation between the effectiveness of a buffer. buffers are characterized by their ph range and buffer capacity.. Difference Between Buffer And Titration.
From www.vrogue.co
What Is Titration And How Does It Work vrogue.co Difference Between Buffer And Titration why are buffers important in our bodies and in nature? By this point, you are familiar with the concepts of acids and bases, and that. titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. buffers are characterized by their ph range and buffer capacity. a titration. Difference Between Buffer And Titration.
From www.slideserve.com
PPT Unit 122 PowerPoint Presentation, free download ID1994619 Difference Between Buffer And Titration buffers are characterized by their ph range and buffer capacity. There is a strong correlation between the effectiveness of a buffer. By this point, you are familiar with the concepts of acids and bases, and that. the relationship between titrations and buffers. titration is a procedure used in chemistry in order to determine the molarity of an. Difference Between Buffer And Titration.
From brunofuga.adv.br
Difference Between AcidBase Titration And Redox Titration, 50 OFF Difference Between Buffer And Titration By this point, you are familiar with the concepts of acids and bases, and that. the relationship between titrations and buffers. a titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. why are buffers important in our bodies and in nature? buffers. Difference Between Buffer And Titration.
From exyfxaqfh.blob.core.windows.net
Titration Practice Problems With Answers Pdf at Jessica Frazier blog Difference Between Buffer And Titration There is a strong correlation between the effectiveness of a buffer. the relationship between titrations and buffers. a titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. titration is a procedure used in chemistry in order to determine the molarity of an acid. Difference Between Buffer And Titration.
From relationshipbetween.com
Difference Between Buffer Action And Buffer Capacity Relationship Between Difference Between Buffer And Titration buffers are characterized by their ph range and buffer capacity. By this point, you are familiar with the concepts of acids and bases, and that. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. There is a strong correlation between the. Difference Between Buffer And Titration.
From www.differencebetween.com
Difference Between pH and Buffer Compare the Difference Between Difference Between Buffer And Titration There is a strong correlation between the effectiveness of a buffer. a titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with. Difference Between Buffer And Titration.
From www.differencebetween.com
Difference Between Complexometric and Redox Titration Compare the Difference Between Buffer And Titration A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. By this point, you are familiar with the concepts of acids and bases, and that. a titration curve is a graph that relates the change in ph of an acidic or basic. Difference Between Buffer And Titration.
From www.differencebetween.com
Difference Between AcidBase Titration and Redox Titration Compare Difference Between Buffer And Titration titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. the relationship between titrations and buffers. why are buffers important in. Difference Between Buffer And Titration.
From www.differencebetween.com
Difference Between Titration and Back Titration Compare the Difference Between Buffer And Titration a titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. There is a strong correlation between the effectiveness of a buffer. why are buffers important in our bodies and in nature? titration is a procedure used in chemistry in order to determine the. Difference Between Buffer And Titration.
From www.slideshare.net
Ch 18 buffers Difference Between Buffer And Titration buffers are characterized by their ph range and buffer capacity. A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. a. Difference Between Buffer And Titration.
From giorjuuhy.blob.core.windows.net
Titration Curve With Points at Geneva Sampson blog Difference Between Buffer And Titration There is a strong correlation between the effectiveness of a buffer. buffers are characterized by their ph range and buffer capacity. a titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. By this point, you are familiar with the concepts of acids and bases,. Difference Between Buffer And Titration.
From www.scribd.com
Buffer Solution and TITraTION1 PDF Buffer Solution Acid Difference Between Buffer And Titration titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. why are buffers important in our bodies and in nature? By this point, you are familiar with the concepts of acids and bases, and that. a titration curve is a graph that relates the change in ph of. Difference Between Buffer And Titration.
From www.differencebetween.com
Difference Between Buffer Action and Buffer Capacity Compare the Difference Between Buffer And Titration A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. a titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. By this point, you are familiar with the. Difference Between Buffer And Titration.
From byjus.com
Buffer Region What is a Buffer Region, Relationship between Titration Difference Between Buffer And Titration There is a strong correlation between the effectiveness of a buffer. titration is a procedure used in chemistry in order to determine the molarity of an acid or a base. buffers are characterized by their ph range and buffer capacity. By this point, you are familiar with the concepts of acids and bases, and that. a titration. Difference Between Buffer And Titration.
From mungfali.com
Phosphate Titration Curve Difference Between Buffer And Titration the relationship between titrations and buffers. why are buffers important in our bodies and in nature? A chemical reaction is set up between a known volume of a solution of unknown concentration and a known volume of a solution with a known concentration. There is a strong correlation between the effectiveness of a buffer. titration is a. Difference Between Buffer And Titration.
From mmerevise.co.uk
pH Curves Questions and Revision MME Difference Between Buffer And Titration why are buffers important in our bodies and in nature? the relationship between titrations and buffers. a titration curve is a graph that relates the change in ph of an acidic or basic solution to the volume of added titrant. buffers are characterized by their ph range and buffer capacity. By this point, you are familiar. Difference Between Buffer And Titration.