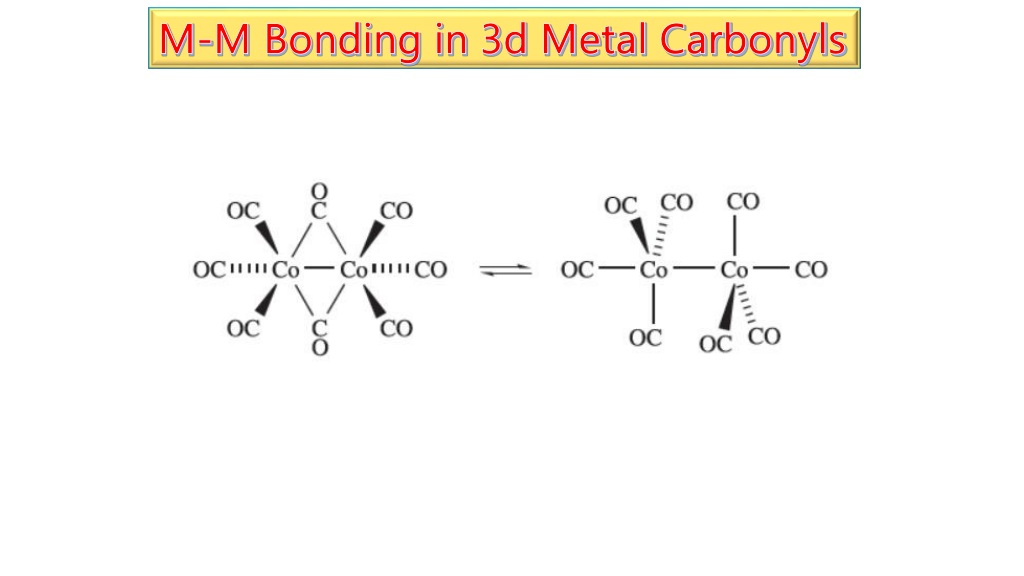
Bonding In Metal Carbonyls Ppt . The document discusses coordination compounds and organometallic compounds. In the 13c nmr spectrum, coordinated carbonyl ligands typically appear in the range of 180 to 250 ppm. It describes bonding in metal carbonyls, including σ and π bonding between the. The co ligand usually binds via the carbon atom to the metal. Substitution reactions of metal carbonyls frequently indicate differences in bonding characteristics of ligands. Furthermore, metal carbonyls are one of. It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples like nickel tetracarbonyl and iron pentacarbonyl.
from www.slideserve.com
Substitution reactions of metal carbonyls frequently indicate differences in bonding characteristics of ligands. It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples like nickel tetracarbonyl and iron pentacarbonyl. The document discusses coordination compounds and organometallic compounds. The co ligand usually binds via the carbon atom to the metal. It describes bonding in metal carbonyls, including σ and π bonding between the. Furthermore, metal carbonyls are one of. In the 13c nmr spectrum, coordinated carbonyl ligands typically appear in the range of 180 to 250 ppm.
PPT MM Bonding in Transition M etal Complexes PowerPoint Presentation ID9684527
Bonding In Metal Carbonyls Ppt The document discusses coordination compounds and organometallic compounds. The document discusses coordination compounds and organometallic compounds. Substitution reactions of metal carbonyls frequently indicate differences in bonding characteristics of ligands. The co ligand usually binds via the carbon atom to the metal. It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples like nickel tetracarbonyl and iron pentacarbonyl. It describes bonding in metal carbonyls, including σ and π bonding between the. In the 13c nmr spectrum, coordinated carbonyl ligands typically appear in the range of 180 to 250 ppm. Furthermore, metal carbonyls are one of.
From www.slideserve.com
PPT Lecture 20. An introduction to organometallic chemistry PowerPoint Presentation ID794384 Bonding In Metal Carbonyls Ppt It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples like nickel tetracarbonyl and iron pentacarbonyl. Furthermore, metal carbonyls are one of. The co ligand usually binds via the carbon atom to the metal. Substitution reactions of metal carbonyls frequently indicate differences in bonding characteristics of ligands. In the 13c nmr spectrum, coordinated carbonyl ligands. Bonding In Metal Carbonyls Ppt.
From www.slideserve.com
PPT Lecture 4 Transition MetAL Organometallics LIGANDS PowerPoint Presentation ID2150776 Bonding In Metal Carbonyls Ppt It describes bonding in metal carbonyls, including σ and π bonding between the. Substitution reactions of metal carbonyls frequently indicate differences in bonding characteristics of ligands. It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples like nickel tetracarbonyl and iron pentacarbonyl. Furthermore, metal carbonyls are one of. The document discusses coordination compounds and organometallic. Bonding In Metal Carbonyls Ppt.
From www.slideserve.com
PPT Carbonyl Ligands C≡O PowerPoint Presentation, free download ID854909 Bonding In Metal Carbonyls Ppt The co ligand usually binds via the carbon atom to the metal. The document discusses coordination compounds and organometallic compounds. It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples like nickel tetracarbonyl and iron pentacarbonyl. In the 13c nmr spectrum, coordinated carbonyl ligands typically appear in the range of 180 to 250 ppm. Furthermore,. Bonding In Metal Carbonyls Ppt.
From www.slideserve.com
PPT Organometallic Chemistry PowerPoint Presentation, free download ID2085734 Bonding In Metal Carbonyls Ppt Substitution reactions of metal carbonyls frequently indicate differences in bonding characteristics of ligands. Furthermore, metal carbonyls are one of. The co ligand usually binds via the carbon atom to the metal. It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples like nickel tetracarbonyl and iron pentacarbonyl. The document discusses coordination compounds and organometallic compounds.. Bonding In Metal Carbonyls Ppt.
From www.slideserve.com
PPT Lecture 20. An introduction to organometallic chemistry PowerPoint Presentation ID794384 Bonding In Metal Carbonyls Ppt Substitution reactions of metal carbonyls frequently indicate differences in bonding characteristics of ligands. The co ligand usually binds via the carbon atom to the metal. The document discusses coordination compounds and organometallic compounds. In the 13c nmr spectrum, coordinated carbonyl ligands typically appear in the range of 180 to 250 ppm. It discusses how metal carbonyls are formed from transition. Bonding In Metal Carbonyls Ppt.
From www.slideserve.com
PPT MCO and MPR 3 Bonds PowerPoint Presentation, free download ID2328238 Bonding In Metal Carbonyls Ppt Furthermore, metal carbonyls are one of. Substitution reactions of metal carbonyls frequently indicate differences in bonding characteristics of ligands. The document discusses coordination compounds and organometallic compounds. It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples like nickel tetracarbonyl and iron pentacarbonyl. The co ligand usually binds via the carbon atom to the metal.. Bonding In Metal Carbonyls Ppt.
From www.slideserve.com
PPT Metal Carbonyls PowerPoint Presentation, free download ID9724163 Bonding In Metal Carbonyls Ppt Furthermore, metal carbonyls are one of. In the 13c nmr spectrum, coordinated carbonyl ligands typically appear in the range of 180 to 250 ppm. It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples like nickel tetracarbonyl and iron pentacarbonyl. It describes bonding in metal carbonyls, including σ and π bonding between the. Substitution reactions. Bonding In Metal Carbonyls Ppt.
From www.slideserve.com
PPT Carbonyl Compounds PowerPoint Presentation, free download ID2821851 Bonding In Metal Carbonyls Ppt It describes bonding in metal carbonyls, including σ and π bonding between the. It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples like nickel tetracarbonyl and iron pentacarbonyl. The document discusses coordination compounds and organometallic compounds. The co ligand usually binds via the carbon atom to the metal. Furthermore, metal carbonyls are one of.. Bonding In Metal Carbonyls Ppt.
From www.youtube.com
Metal Carbonyls Bonding Structure YouTube Bonding In Metal Carbonyls Ppt It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples like nickel tetracarbonyl and iron pentacarbonyl. Furthermore, metal carbonyls are one of. The document discusses coordination compounds and organometallic compounds. It describes bonding in metal carbonyls, including σ and π bonding between the. In the 13c nmr spectrum, coordinated carbonyl ligands typically appear in the. Bonding In Metal Carbonyls Ppt.
From www.slideserve.com
PPT Metal carbonyl and related complexes (dative ligands) PowerPoint Presentation ID3353269 Bonding In Metal Carbonyls Ppt It describes bonding in metal carbonyls, including σ and π bonding between the. Substitution reactions of metal carbonyls frequently indicate differences in bonding characteristics of ligands. The document discusses coordination compounds and organometallic compounds. In the 13c nmr spectrum, coordinated carbonyl ligands typically appear in the range of 180 to 250 ppm. Furthermore, metal carbonyls are one of. It discusses. Bonding In Metal Carbonyls Ppt.
From www.slideserve.com
PPT MCO and MPR 3 Bonds PowerPoint Presentation, free download ID2328238 Bonding In Metal Carbonyls Ppt The document discusses coordination compounds and organometallic compounds. Substitution reactions of metal carbonyls frequently indicate differences in bonding characteristics of ligands. It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples like nickel tetracarbonyl and iron pentacarbonyl. It describes bonding in metal carbonyls, including σ and π bonding between the. The co ligand usually binds. Bonding In Metal Carbonyls Ppt.
From www.slideshare.net
Metal carbonyls PPT Bonding In Metal Carbonyls Ppt The document discusses coordination compounds and organometallic compounds. The co ligand usually binds via the carbon atom to the metal. Substitution reactions of metal carbonyls frequently indicate differences in bonding characteristics of ligands. It describes bonding in metal carbonyls, including σ and π bonding between the. It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and. Bonding In Metal Carbonyls Ppt.
From www.scribd.com
524Chem Metal Carbonyl Cluster Bondingw (1) Cluster Chemistry Coordination Complex Bonding In Metal Carbonyls Ppt Furthermore, metal carbonyls are one of. The co ligand usually binds via the carbon atom to the metal. The document discusses coordination compounds and organometallic compounds. Substitution reactions of metal carbonyls frequently indicate differences in bonding characteristics of ligands. It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples like nickel tetracarbonyl and iron pentacarbonyl.. Bonding In Metal Carbonyls Ppt.
From www.slideshare.net
Metal carbonyls PPT Bonding In Metal Carbonyls Ppt The document discusses coordination compounds and organometallic compounds. It describes bonding in metal carbonyls, including σ and π bonding between the. In the 13c nmr spectrum, coordinated carbonyl ligands typically appear in the range of 180 to 250 ppm. It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples like nickel tetracarbonyl and iron pentacarbonyl.. Bonding In Metal Carbonyls Ppt.
From www.slideserve.com
PPT Lecture 31 Structure and reactivity of organometallic compounds 1) Bonding and reactivity Bonding In Metal Carbonyls Ppt In the 13c nmr spectrum, coordinated carbonyl ligands typically appear in the range of 180 to 250 ppm. It describes bonding in metal carbonyls, including σ and π bonding between the. The co ligand usually binds via the carbon atom to the metal. The document discusses coordination compounds and organometallic compounds. It discusses how metal carbonyls are formed from transition. Bonding In Metal Carbonyls Ppt.
From www.slideserve.com
PPT Lecture 31 Structure and reactivity of organometallic compounds 1) Bonding and reactivity Bonding In Metal Carbonyls Ppt It describes bonding in metal carbonyls, including σ and π bonding between the. Furthermore, metal carbonyls are one of. Substitution reactions of metal carbonyls frequently indicate differences in bonding characteristics of ligands. In the 13c nmr spectrum, coordinated carbonyl ligands typically appear in the range of 180 to 250 ppm. The co ligand usually binds via the carbon atom to. Bonding In Metal Carbonyls Ppt.
From www.doubtnut.com
Explain bonding in metal carbonyls. Bonding In Metal Carbonyls Ppt Furthermore, metal carbonyls are one of. The document discusses coordination compounds and organometallic compounds. The co ligand usually binds via the carbon atom to the metal. In the 13c nmr spectrum, coordinated carbonyl ligands typically appear in the range of 180 to 250 ppm. It describes bonding in metal carbonyls, including σ and π bonding between the. Substitution reactions of. Bonding In Metal Carbonyls Ppt.
From www.studocu.com
Structure of Binuclear and Polynuclear Metal Carbonyls Structure and bonding of binuclear Bonding In Metal Carbonyls Ppt The document discusses coordination compounds and organometallic compounds. The co ligand usually binds via the carbon atom to the metal. It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples like nickel tetracarbonyl and iron pentacarbonyl. It describes bonding in metal carbonyls, including σ and π bonding between the. Furthermore, metal carbonyls are one of.. Bonding In Metal Carbonyls Ppt.
From slideplayer.com
Coordination compounds and Organometallics ppt download Bonding In Metal Carbonyls Ppt In the 13c nmr spectrum, coordinated carbonyl ligands typically appear in the range of 180 to 250 ppm. The document discusses coordination compounds and organometallic compounds. Substitution reactions of metal carbonyls frequently indicate differences in bonding characteristics of ligands. It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples like nickel tetracarbonyl and iron pentacarbonyl.. Bonding In Metal Carbonyls Ppt.
From www.youtube.com
Chemistry 12 Chapter 9 Bonding in Metal Carbonyls Importance & Application of Coordination Bonding In Metal Carbonyls Ppt It describes bonding in metal carbonyls, including σ and π bonding between the. The co ligand usually binds via the carbon atom to the metal. Substitution reactions of metal carbonyls frequently indicate differences in bonding characteristics of ligands. Furthermore, metal carbonyls are one of. It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples like. Bonding In Metal Carbonyls Ppt.
From www.studypool.com
SOLUTION Metal carbonyl structure and bonding bs chemistry Studypool Bonding In Metal Carbonyls Ppt Substitution reactions of metal carbonyls frequently indicate differences in bonding characteristics of ligands. The co ligand usually binds via the carbon atom to the metal. Furthermore, metal carbonyls are one of. It describes bonding in metal carbonyls, including σ and π bonding between the. In the 13c nmr spectrum, coordinated carbonyl ligands typically appear in the range of 180 to. Bonding In Metal Carbonyls Ppt.
From www.slideshare.net
Metal carbonyls PPT Bonding In Metal Carbonyls Ppt The co ligand usually binds via the carbon atom to the metal. It describes bonding in metal carbonyls, including σ and π bonding between the. Furthermore, metal carbonyls are one of. The document discusses coordination compounds and organometallic compounds. In the 13c nmr spectrum, coordinated carbonyl ligands typically appear in the range of 180 to 250 ppm. It discusses how. Bonding In Metal Carbonyls Ppt.
From www.dalalinstitute.com
Metal Carbonyls Structure and Bonding Dalal Institute CHEMISTRY Bonding In Metal Carbonyls Ppt The co ligand usually binds via the carbon atom to the metal. It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples like nickel tetracarbonyl and iron pentacarbonyl. In the 13c nmr spectrum, coordinated carbonyl ligands typically appear in the range of 180 to 250 ppm. It describes bonding in metal carbonyls, including σ and. Bonding In Metal Carbonyls Ppt.
From www.slideserve.com
PPT MM Bonding in Transition M etal Complexes PowerPoint Presentation ID9684527 Bonding In Metal Carbonyls Ppt Furthermore, metal carbonyls are one of. Substitution reactions of metal carbonyls frequently indicate differences in bonding characteristics of ligands. The co ligand usually binds via the carbon atom to the metal. It describes bonding in metal carbonyls, including σ and π bonding between the. It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples like. Bonding In Metal Carbonyls Ppt.
From www.slideserve.com
PPT Metal Carbonyls PowerPoint Presentation, free download ID9724163 Bonding In Metal Carbonyls Ppt The document discusses coordination compounds and organometallic compounds. In the 13c nmr spectrum, coordinated carbonyl ligands typically appear in the range of 180 to 250 ppm. It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples like nickel tetracarbonyl and iron pentacarbonyl. It describes bonding in metal carbonyls, including σ and π bonding between the.. Bonding In Metal Carbonyls Ppt.
From www.slideserve.com
PPT Lecture 31 Structure and reactivity of organometallic compounds 1) Bonding and reactivity Bonding In Metal Carbonyls Ppt In the 13c nmr spectrum, coordinated carbonyl ligands typically appear in the range of 180 to 250 ppm. It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples like nickel tetracarbonyl and iron pentacarbonyl. The co ligand usually binds via the carbon atom to the metal. It describes bonding in metal carbonyls, including σ and. Bonding In Metal Carbonyls Ppt.
From www.slideserve.com
PPT Metal Carbonyls PowerPoint Presentation, free download ID9724163 Bonding In Metal Carbonyls Ppt Furthermore, metal carbonyls are one of. It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples like nickel tetracarbonyl and iron pentacarbonyl. The document discusses coordination compounds and organometallic compounds. It describes bonding in metal carbonyls, including σ and π bonding between the. The co ligand usually binds via the carbon atom to the metal.. Bonding In Metal Carbonyls Ppt.
From www.slideserve.com
PPT CHEM 522 Chapter 04 PowerPoint Presentation, free download ID6797297 Bonding In Metal Carbonyls Ppt Furthermore, metal carbonyls are one of. Substitution reactions of metal carbonyls frequently indicate differences in bonding characteristics of ligands. The document discusses coordination compounds and organometallic compounds. It describes bonding in metal carbonyls, including σ and π bonding between the. In the 13c nmr spectrum, coordinated carbonyl ligands typically appear in the range of 180 to 250 ppm. It discusses. Bonding In Metal Carbonyls Ppt.
From www.slideserve.com
PPT Lecture 4 Transition MetAL Organometallics LIGANDS PowerPoint Presentation ID2150776 Bonding In Metal Carbonyls Ppt It describes bonding in metal carbonyls, including σ and π bonding between the. In the 13c nmr spectrum, coordinated carbonyl ligands typically appear in the range of 180 to 250 ppm. It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples like nickel tetracarbonyl and iron pentacarbonyl. The co ligand usually binds via the carbon. Bonding In Metal Carbonyls Ppt.
From www.youtube.com
Metal Carbonyls Part2 Back Bonding MOT Chemistry CarbonMonoxide ZCC YouTube Bonding In Metal Carbonyls Ppt It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples like nickel tetracarbonyl and iron pentacarbonyl. The co ligand usually binds via the carbon atom to the metal. In the 13c nmr spectrum, coordinated carbonyl ligands typically appear in the range of 180 to 250 ppm. Substitution reactions of metal carbonyls frequently indicate differences in. Bonding In Metal Carbonyls Ppt.
From www.slideserve.com
PPT MCO and MPR 3 Bonds PowerPoint Presentation, free download ID2328238 Bonding In Metal Carbonyls Ppt The co ligand usually binds via the carbon atom to the metal. In the 13c nmr spectrum, coordinated carbonyl ligands typically appear in the range of 180 to 250 ppm. Furthermore, metal carbonyls are one of. It describes bonding in metal carbonyls, including σ and π bonding between the. It discusses how metal carbonyls are formed from transition metals and. Bonding In Metal Carbonyls Ppt.
From www.slideserve.com
PPT Metal Carbonyls PowerPoint Presentation, free download ID9724163 Bonding In Metal Carbonyls Ppt Furthermore, metal carbonyls are one of. The document discusses coordination compounds and organometallic compounds. In the 13c nmr spectrum, coordinated carbonyl ligands typically appear in the range of 180 to 250 ppm. Substitution reactions of metal carbonyls frequently indicate differences in bonding characteristics of ligands. It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples. Bonding In Metal Carbonyls Ppt.
From slideplayer.com
Lecture 5 pAcceptor Ligands and Biology CO, N2 and O2 complexes ppt download Bonding In Metal Carbonyls Ppt The document discusses coordination compounds and organometallic compounds. The co ligand usually binds via the carbon atom to the metal. Furthermore, metal carbonyls are one of. It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples like nickel tetracarbonyl and iron pentacarbonyl. It describes bonding in metal carbonyls, including σ and π bonding between the.. Bonding In Metal Carbonyls Ppt.
From www.slideserve.com
PPT Metal Carbonyls PowerPoint Presentation, free download ID9724163 Bonding In Metal Carbonyls Ppt Furthermore, metal carbonyls are one of. Substitution reactions of metal carbonyls frequently indicate differences in bonding characteristics of ligands. It discusses how metal carbonyls are formed from transition metals and carbon monoxide, and examples like nickel tetracarbonyl and iron pentacarbonyl. In the 13c nmr spectrum, coordinated carbonyl ligands typically appear in the range of 180 to 250 ppm. The co. Bonding In Metal Carbonyls Ppt.
From www.slideserve.com
PPT Metal Carbonyls PowerPoint Presentation, free download ID9724163 Bonding In Metal Carbonyls Ppt Substitution reactions of metal carbonyls frequently indicate differences in bonding characteristics of ligands. Furthermore, metal carbonyls are one of. In the 13c nmr spectrum, coordinated carbonyl ligands typically appear in the range of 180 to 250 ppm. The co ligand usually binds via the carbon atom to the metal. The document discusses coordination compounds and organometallic compounds. It describes bonding. Bonding In Metal Carbonyls Ppt.