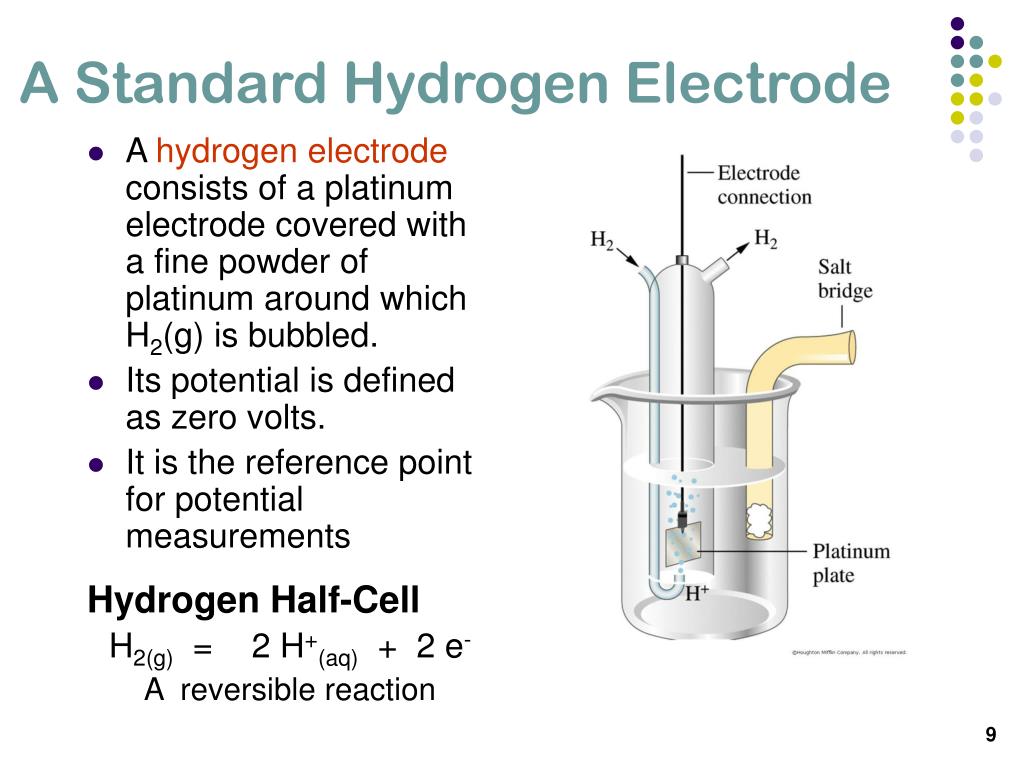
Standard Hydrogen Electrode Platinum Used . The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the fundamentals of both the standard and reversible. What is going on in this process? Platinum is used because it is inert and does not react much with hydrogen. The structure of the standard. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. First an initial discharge allows.
from www.slideserve.com
Platinum is used because it is inert and does not react much with hydrogen. The structure of the standard. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. What is going on in this process? First an initial discharge allows. The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the fundamentals of both the standard and reversible.
PPT Electrochemistry PowerPoint Presentation, free download ID3975392
Standard Hydrogen Electrode Platinum Used Platinum is used because it is inert and does not react much with hydrogen. What is going on in this process? The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. The structure of the standard. The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the fundamentals of both the standard and reversible. First an initial discharge allows. Platinum is used because it is inert and does not react much with hydrogen.
From stock.adobe.com
Standard hydrogen electrode diagram. Scientific vector illustration isolated on white background Standard Hydrogen Electrode Platinum Used First an initial discharge allows. The structure of the standard. Platinum is used because it is inert and does not react much with hydrogen. What is going on in this process? The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the fundamentals of both the standard and reversible. The emf measured when a metal. Standard Hydrogen Electrode Platinum Used.
From app.pandai.org
Standard Electrode Potential Standard Hydrogen Electrode Platinum Used The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. Platinum is used because it is inert and does not react much with hydrogen. The structure of the standard. The viewpoint addresses some of the gaps that exists. Standard Hydrogen Electrode Platinum Used.
From stock.adobe.com
Vetor de A Standard Hydrogen Electrode or SHE is an electrode that scientists use as a reference Standard Hydrogen Electrode Platinum Used First an initial discharge allows. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. What is going on in this process? The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard. Standard Hydrogen Electrode Platinum Used.
From royalweldingwires.com
How Standard Hydrogen Electrode Works Best Guide Standard Hydrogen Electrode Platinum Used First an initial discharge allows. Platinum is used because it is inert and does not react much with hydrogen. The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the fundamentals of both the standard and reversible. The structure of the standard. What is going on in this process? The standard hydrogen electrode (she) is. Standard Hydrogen Electrode Platinum Used.
From www.numerade.com
SOLVED What is the function of platinum in the standard hydrogen electrode? Standard Hydrogen Electrode Platinum Used First an initial discharge allows. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the fundamentals of both the standard and reversible.. Standard Hydrogen Electrode Platinum Used.
From saylordotorg.github.io
Electrochemistry Standard Hydrogen Electrode Platinum Used The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. First an initial discharge allows. The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the fundamentals of both the standard and reversible. Platinum is used because it is inert and does not react much. Standard Hydrogen Electrode Platinum Used.
From www.slideserve.com
PPT Standard hydrogen electrode PowerPoint Presentation, free download ID9151944 Standard Hydrogen Electrode Platinum Used The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. Platinum is used because it is inert and does not react much with hydrogen. The structure of the standard. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known. Standard Hydrogen Electrode Platinum Used.
From 2012books.lardbucket.org
Standard Potentials Standard Hydrogen Electrode Platinum Used The structure of the standard. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the fundamentals of both the standard and reversible. What is going on in this process? The emf measured when a. Standard Hydrogen Electrode Platinum Used.
From www.youtube.com
Standard Hydrogen Electrode Demonstration YouTube Standard Hydrogen Electrode Platinum Used The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. Platinum is used because it is inert and does not react much with hydrogen. The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the fundamentals of both the standard and reversible. First an initial. Standard Hydrogen Electrode Platinum Used.
From gaskatel.de
Principles of the pHvalue measurement with hydrogen electrodes Gaskatel Standard Hydrogen Electrode Platinum Used The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the fundamentals of both the standard and reversible. The structure of the standard. What is going on in this process? The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode. Standard Hydrogen Electrode Platinum Used.
From www.slideserve.com
PPT Chapter 21 Electrochemistry PowerPoint Presentation, free download ID5405180 Standard Hydrogen Electrode Platinum Used What is going on in this process? The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the fundamentals of both the standard and reversible. Platinum is used because it is inert and does not react much with hydrogen. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode. Standard Hydrogen Electrode Platinum Used.
From slideplayer.com
KNOCKHARDY PUBLISHING ppt download Standard Hydrogen Electrode Platinum Used The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. What is going on in this process? The structure of the standard. The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the. Standard Hydrogen Electrode Platinum Used.
From monomole.com
Standard hydrogen electrode Mono Mole Standard Hydrogen Electrode Platinum Used First an initial discharge allows. Platinum is used because it is inert and does not react much with hydrogen. The structure of the standard. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The viewpoint addresses some. Standard Hydrogen Electrode Platinum Used.
From www.savemyexams.com
The Hydrogen Electrode (HL) HL IB Chemistry Revision Notes 2025 Standard Hydrogen Electrode Platinum Used The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. First an initial discharge allows. What is going on in this process? The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the fundamentals of both the standard and reversible. The emf measured when a. Standard Hydrogen Electrode Platinum Used.
From chemistnotes.com
Standard hydrogen electrode(SHE) Definition, diagram, application Chemistry Notes Standard Hydrogen Electrode Platinum Used First an initial discharge allows. The structure of the standard. Platinum is used because it is inert and does not react much with hydrogen. The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the fundamentals of both the standard and reversible. The standard hydrogen electrode (she) is universally used for this purpose and is. Standard Hydrogen Electrode Platinum Used.
From brainly.in
What is standard hydrogen electrode? How is it prepared? Explain with labelled diagram? Brainly.in Standard Hydrogen Electrode Platinum Used Platinum is used because it is inert and does not react much with hydrogen. The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the fundamentals of both the standard and reversible. First an initial discharge allows. What is going on in this process? The standard hydrogen electrode (she) is universally used for this purpose. Standard Hydrogen Electrode Platinum Used.
From stock.adobe.com
A Standard Hydrogen Electrode (SHE) is an electrode that scientists use as a reference electrode Standard Hydrogen Electrode Platinum Used The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. Platinum is used because it is inert and. Standard Hydrogen Electrode Platinum Used.
From kindle-tech.com
Understanding Electrodes And Electrochemical Cells Kintek Solution Standard Hydrogen Electrode Platinum Used The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the fundamentals of both the standard and reversible. What is going on in this process? The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal /. Standard Hydrogen Electrode Platinum Used.
From www.numerade.com
SOLVED 14. Why'` lis platinum included in the hydrogen electrode, as shown below? Pt A Platinum Standard Hydrogen Electrode Platinum Used First an initial discharge allows. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. What is going on in this process? The structure of the standard. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. Standard Hydrogen Electrode Platinum Used.
From mmerevise.co.uk
Electrochemical Cells MME Standard Hydrogen Electrode Platinum Used The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the fundamentals of both the standard and reversible. First an initial discharge allows. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. Platinum is used because it is inert and does not react much. Standard Hydrogen Electrode Platinum Used.
From royalweldingwires.com
Standard Hydrogen Electrode Advantages and Disadvantages Standard Hydrogen Electrode Platinum Used The structure of the standard. The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the fundamentals of both the standard and reversible. What is going on in this process? The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. The emf measured when a. Standard Hydrogen Electrode Platinum Used.
From www.lambdasystem.pl
Hydrogen reference electrode Electrodes ELECTROCHEMISTRY ACCESSORIES Electrochemistry Standard Hydrogen Electrode Platinum Used Platinum is used because it is inert and does not react much with hydrogen. The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the fundamentals of both the standard and reversible. First an initial discharge allows. The structure of the standard. What is going on in this process? The standard hydrogen electrode (she) is. Standard Hydrogen Electrode Platinum Used.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID3975392 Standard Hydrogen Electrode Platinum Used The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The structure of the standard. What is going on in this process? The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the. Standard Hydrogen Electrode Platinum Used.
From www.youtube.com
Standard Hydrogen Electrode (SHE)/ Primary reference electrode Gas electrode YouTube Standard Hydrogen Electrode Platinum Used First an initial discharge allows. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the fundamentals of both the standard and reversible.. Standard Hydrogen Electrode Platinum Used.
From dohsdocfeco.blob.core.windows.net
Facts About Standard Hydrogen Electrode at Troy Williams blog Standard Hydrogen Electrode Platinum Used First an initial discharge allows. What is going on in this process? Platinum is used because it is inert and does not react much with hydrogen. The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the fundamentals of both the standard and reversible. The emf measured when a metal / metal ion electrode is. Standard Hydrogen Electrode Platinum Used.
From www.numerade.com
SOLVEDA Reference Point The Standard Hydrogen Electrode What is the function of platinum in Standard Hydrogen Electrode Platinum Used The structure of the standard. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. Platinum is used because it is inert and does not react much with hydrogen. First an initial discharge allows. The viewpoint addresses some. Standard Hydrogen Electrode Platinum Used.
From www.youtube.com
19.1 Standard hydrogen electrode (HL) YouTube Standard Hydrogen Electrode Platinum Used The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the fundamentals of both the standard and reversible. The structure of the standard. Platinum is used because it is inert and does not react much with hydrogen. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of. Standard Hydrogen Electrode Platinum Used.
From cider.uoregon.edu
Standard Hydrogen Electrode Demonstration and AACT Simulation CIDER Standard Hydrogen Electrode Platinum Used The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the fundamentals of both the standard and reversible. First an initial discharge allows. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. Platinum is used because it is inert and does not react much. Standard Hydrogen Electrode Platinum Used.
From scienceinfo.com
What is Standard Hydrogen Electrode? Standard Hydrogen Electrode Platinum Used First an initial discharge allows. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the fundamentals of both the standard and reversible. Platinum is used because it is inert and does not react much. Standard Hydrogen Electrode Platinum Used.
From question.pandai.org
Standard Electrode Potential Standard Hydrogen Electrode Platinum Used The structure of the standard. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. First an initial discharge allows. The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the fundamentals of. Standard Hydrogen Electrode Platinum Used.
From cider.uoregon.edu
Standard Hydrogen Electrode Demonstration and AACT Simulation CIDER Standard Hydrogen Electrode Platinum Used The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the fundamentals of both the standard and reversible. Platinum is used because it is inert and does not react much with hydrogen. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. The structure of. Standard Hydrogen Electrode Platinum Used.
From pubs.acs.org
Standard and Reversible Hydrogen Electrodes Theory, Design, Operation, and Applications ACS Standard Hydrogen Electrode Platinum Used First an initial discharge allows. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. Platinum is used because it is inert and does not react much with hydrogen. The structure of the standard. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode. Standard Hydrogen Electrode Platinum Used.
From www.numerade.com
SOLVEDPlatinum is very expensive, so why is it used in standard hydrogen standard electrodes Standard Hydrogen Electrode Platinum Used The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. What is going on in this process? The structure of the standard. First an initial discharge allows. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the. Standard Hydrogen Electrode Platinum Used.
From byjus.com
Standard Hydrogen Electrode Definition, Construction, and Labelled Diagram Standard Hydrogen Electrode Platinum Used What is going on in this process? The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. Platinum is used because it is inert and does not react much with hydrogen. The viewpoint addresses some of the gaps. Standard Hydrogen Electrode Platinum Used.
From gaskatel.de
Reference electrode Gaskatel Standard Hydrogen Electrode Platinum Used First an initial discharge allows. The standard hydrogen electrode (she) is universally used for this purpose and is assigned a standard potential of 0 v. Platinum is used because it is inert and does not react much with hydrogen. The structure of the standard. The viewpoint addresses some of the gaps that exists in the electrochemistry literature and explains the. Standard Hydrogen Electrode Platinum Used.