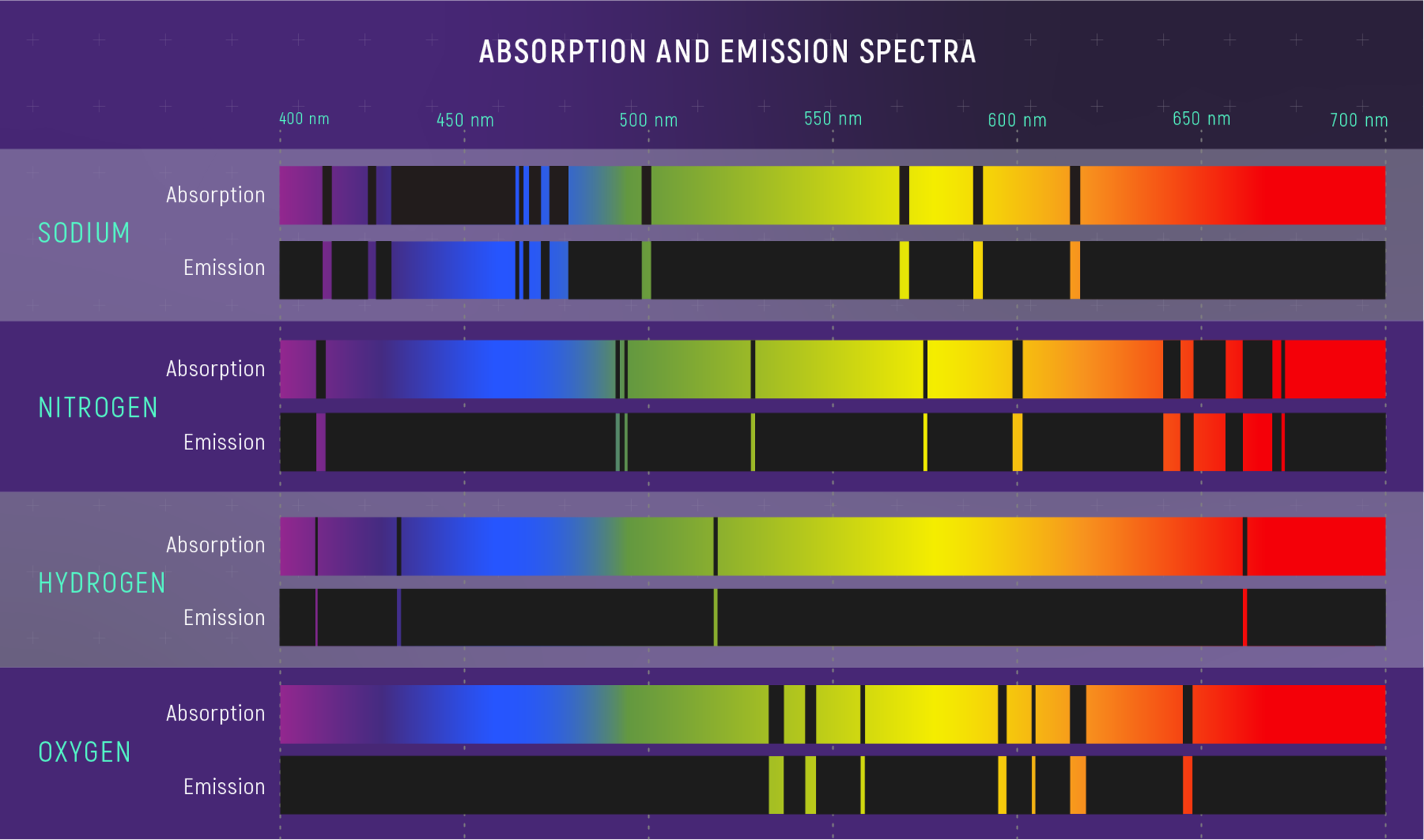
Emission Spectra Unknown Element . the identification of unknown elements requires a more complete identification of all spectral lines in the. the emission spectra of various atoms. the set of individual colors emitted by an element is called its spectrum. each element has its own unique absorption spectra, just like it has a unique emission spectra. This means they can be used to. Since the spectrum of each element is unique,. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. elements in an unknown spectrum can be identified by comparing it with the spectrum of the known elements. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it.
from www.esa.int
the identification of unknown elements requires a more complete identification of all spectral lines in the. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it. the set of individual colors emitted by an element is called its spectrum. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. each element has its own unique absorption spectra, just like it has a unique emission spectra. the emission spectra of various atoms. elements in an unknown spectrum can be identified by comparing it with the spectrum of the known elements. This means they can be used to. Since the spectrum of each element is unique,.
ESA Absorption and emission spectra of various elements
Emission Spectra Unknown Element each element has its own unique absorption spectra, just like it has a unique emission spectra. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. elements in an unknown spectrum can be identified by comparing it with the spectrum of the known elements. the identification of unknown elements requires a more complete identification of all spectral lines in the. the emission spectra of various atoms. each element has its own unique absorption spectra, just like it has a unique emission spectra. the set of individual colors emitted by an element is called its spectrum. Since the spectrum of each element is unique,. This means they can be used to. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it.
From universetoday.com
Spectroscopy The Key to Humanity’s Future in Space Emission Spectra Unknown Element each element has its own unique absorption spectra, just like it has a unique emission spectra. elements in an unknown spectrum can be identified by comparing it with the spectrum of the known elements. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to. Emission Spectra Unknown Element.
From physics.stackexchange.com
optics How do astronomers identify different elements from the Emission Spectra Unknown Element the emission spectra of various atoms. the set of individual colors emitted by an element is called its spectrum. each element has its own unique absorption spectra, just like it has a unique emission spectra. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is. Emission Spectra Unknown Element.
From slidetodoc.com
Atomic Emission Spectra Spectrum Visible Spectrum White Emission Spectra Unknown Element elements in an unknown spectrum can be identified by comparing it with the spectrum of the known elements. the identification of unknown elements requires a more complete identification of all spectral lines in the. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies. Emission Spectra Unknown Element.
From www.resonancescience.org
What is Resonance and Why is it so Important? Emission Spectra Unknown Element the emission spectra of various atoms. the identification of unknown elements requires a more complete identification of all spectral lines in the. each element has its own unique absorption spectra, just like it has a unique emission spectra. Since the spectrum of each element is unique,. This means they can be used to. elements in an. Emission Spectra Unknown Element.
From www.mrpalermo.com
Bright Line Spectra Mr. Palermo's Flipped Chemistry Classroom Emission Spectra Unknown Element the identification of unknown elements requires a more complete identification of all spectral lines in the. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it. This means they can be used to. the set of individual colors emitted by an. Emission Spectra Unknown Element.
From adawyaf.blogspot.com
Chemistry Grade 9, Atomic Emission Spectra , Introduction Emission Spectra Unknown Element This means they can be used to. elements in an unknown spectrum can be identified by comparing it with the spectrum of the known elements. the emission spectra of various atoms. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it.. Emission Spectra Unknown Element.
From www.sciencephoto.com
HHeHg emission spectra Stock Image C017/7260 Science Photo Library Emission Spectra Unknown Element an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it. the set of individual colors emitted by an element is called its spectrum. the emission spectra of various atoms. the identification of unknown elements requires a more complete identification of. Emission Spectra Unknown Element.
From www.evolving-science.com
Alternative Illumination The Next Generation of MoleculeLevel Emission Spectra Unknown Element the emission spectra of various atoms. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it. the set of individual colors emitted by an element is called its spectrum. This means they can be used to. the emission spectrum (or. Emission Spectra Unknown Element.
From www.chegg.com
Solved Spectroscopy Unknown. The spectra and data provided Emission Spectra Unknown Element Since the spectrum of each element is unique,. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. the set of individual colors emitted by an element is called its spectrum. elements in an unknown spectrum can be identified by comparing it. Emission Spectra Unknown Element.
From www.esa.int
ESA Absorption and emission spectra of various elements Emission Spectra Unknown Element each element has its own unique absorption spectra, just like it has a unique emission spectra. the emission spectra of various atoms. Since the spectrum of each element is unique,. This means they can be used to. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element. Emission Spectra Unknown Element.
From courses.lumenlearning.com
Spectroscopy in Astronomy Astronomy Emission Spectra Unknown Element elements in an unknown spectrum can be identified by comparing it with the spectrum of the known elements. the identification of unknown elements requires a more complete identification of all spectral lines in the. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to. Emission Spectra Unknown Element.
From spiff.rit.edu
Spectrographs and Spectra Emission Spectra Unknown Element Since the spectrum of each element is unique,. the emission spectra of various atoms. each element has its own unique absorption spectra, just like it has a unique emission spectra. the set of individual colors emitted by an element is called its spectrum. the emission spectrum (or line spectrum) of a chemical element is the unique. Emission Spectra Unknown Element.
From www.secretsofuniverse.in
How Do Scientists Determine The Temperature Of The Stars Trillions Of Emission Spectra Unknown Element the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. the set of individual colors emitted by an element is called its spectrum. the identification of unknown elements requires a more complete identification of all spectral lines in the. each element. Emission Spectra Unknown Element.
From brainly.com
below are diagrams for the bright line spectra of four elements and the Emission Spectra Unknown Element Since the spectrum of each element is unique,. This means they can be used to. the set of individual colors emitted by an element is called its spectrum. the emission spectra of various atoms. each element has its own unique absorption spectra, just like it has a unique emission spectra. elements in an unknown spectrum can. Emission Spectra Unknown Element.
From studylib.net
Every element has its own characteristic spectral emission Emission Spectra Unknown Element elements in an unknown spectrum can be identified by comparing it with the spectrum of the known elements. each element has its own unique absorption spectra, just like it has a unique emission spectra. the emission spectra of various atoms. the identification of unknown elements requires a more complete identification of all spectral lines in the.. Emission Spectra Unknown Element.
From www.nagwa.com
Question Video Identifying the Emission Spectrum Corresponding to an Emission Spectra Unknown Element This means they can be used to. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. each element has its own unique absorption spectra, just like it has a unique emission spectra. the set of individual colors emitted by an element. Emission Spectra Unknown Element.
From rightmetal.weebly.com
Atomic emission spectrum chemistry definition rightmetal Emission Spectra Unknown Element the set of individual colors emitted by an element is called its spectrum. This means they can be used to. the emission spectra of various atoms. the identification of unknown elements requires a more complete identification of all spectral lines in the. each element has its own unique absorption spectra, just like it has a unique. Emission Spectra Unknown Element.
From www.nagwa.com
Question Video Identifiying a gas using its emission spectrum Nagwa Emission Spectra Unknown Element an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it. Since the spectrum of each element is unique,. the set of individual colors emitted by an element is called its spectrum. the emission spectrum (or line spectrum) of a chemical element. Emission Spectra Unknown Element.
From pages.uoregon.edu
Astronomy 122 Spectroscopy Emission Spectra Unknown Element elements in an unknown spectrum can be identified by comparing it with the spectrum of the known elements. the identification of unknown elements requires a more complete identification of all spectral lines in the. the set of individual colors emitted by an element is called its spectrum. an atomic emission spectrum is the pattern of lines. Emission Spectra Unknown Element.
From www.coursehero.com
[Solved] Figure 1 shows the emission spectra of five substances Emission Spectra Unknown Element the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it. each element has its own unique. Emission Spectra Unknown Element.
From studyandanswers.com
Astudent analyzes the emission spectrum of an unknown element and Emission Spectra Unknown Element each element has its own unique absorption spectra, just like it has a unique emission spectra. This means they can be used to. the set of individual colors emitted by an element is called its spectrum. the emission spectra of various atoms. Since the spectrum of each element is unique,. elements in an unknown spectrum can. Emission Spectra Unknown Element.
From www.chegg.com
Solved The Visible Spectrum 400 450 500 650 700 550 600 Emission Spectra Unknown Element the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. This means they can be used to. the emission spectra of various atoms. Since the spectrum of each element is unique,. each element has its own unique absorption spectra, just like it. Emission Spectra Unknown Element.
From exoszwsiw.blob.core.windows.net
Emission Spectra Stars at Michael Ibarra blog Emission Spectra Unknown Element each element has its own unique absorption spectra, just like it has a unique emission spectra. the set of individual colors emitted by an element is called its spectrum. the emission spectra of various atoms. the identification of unknown elements requires a more complete identification of all spectral lines in the. Since the spectrum of each. Emission Spectra Unknown Element.
From learningzoneteiar09.z14.web.core.windows.net
Atomic Spectra And Its Types Emission Spectra Unknown Element elements in an unknown spectrum can be identified by comparing it with the spectrum of the known elements. each element has its own unique absorption spectra, just like it has a unique emission spectra. the set of individual colors emitted by an element is called its spectrum. the identification of unknown elements requires a more complete. Emission Spectra Unknown Element.
From www.vrogue.co
The Same Concepts Used To Describe The Emission And A vrogue.co Emission Spectra Unknown Element the emission spectra of various atoms. elements in an unknown spectrum can be identified by comparing it with the spectrum of the known elements. the identification of unknown elements requires a more complete identification of all spectral lines in the. an atomic emission spectrum is the pattern of lines formed when light passes through a prism. Emission Spectra Unknown Element.
From umop.net
Visible Spectra of the Elements Emission Spectra Unknown Element This means they can be used to. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it.. Emission Spectra Unknown Element.
From www.chegg.com
Solved Figure 10 emission spectra of five known elements Emission Spectra Unknown Element Since the spectrum of each element is unique,. This means they can be used to. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. the emission spectra of various atoms. the identification of unknown elements requires a more complete identification of. Emission Spectra Unknown Element.
From www.chegg.com
Solved An emission spectrum of an unknown element is Emission Spectra Unknown Element This means they can be used to. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. the identification of unknown elements requires a more complete identification of all spectral lines in the. elements in an unknown spectrum can be identified by. Emission Spectra Unknown Element.
From www.coursehero.com
[Solved] Figure 1 shows the emission spectra of five substances Emission Spectra Unknown Element the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. Since the spectrum of each element is unique,. the identification of unknown elements requires a more complete identification of all spectral lines in the. the set of individual colors emitted by an. Emission Spectra Unknown Element.
From www.vrogue.co
Hydrogen Spectrum Emission Absorption Series Diagram vrogue.co Emission Spectra Unknown Element Since the spectrum of each element is unique,. This means they can be used to. the set of individual colors emitted by an element is called its spectrum. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it. the emission spectra. Emission Spectra Unknown Element.
From www.coursehero.com
[Solved] Figure 1 shows the emission spectra of five substances Emission Spectra Unknown Element the emission spectra of various atoms. the emission spectrum (or line spectrum) of a chemical element is the unique pattern of light obtained when the element is subjected to heat or. an atomic emission spectrum is the pattern of lines formed when light passes through a prism to separate it into the different frequencies of light it.. Emission Spectra Unknown Element.