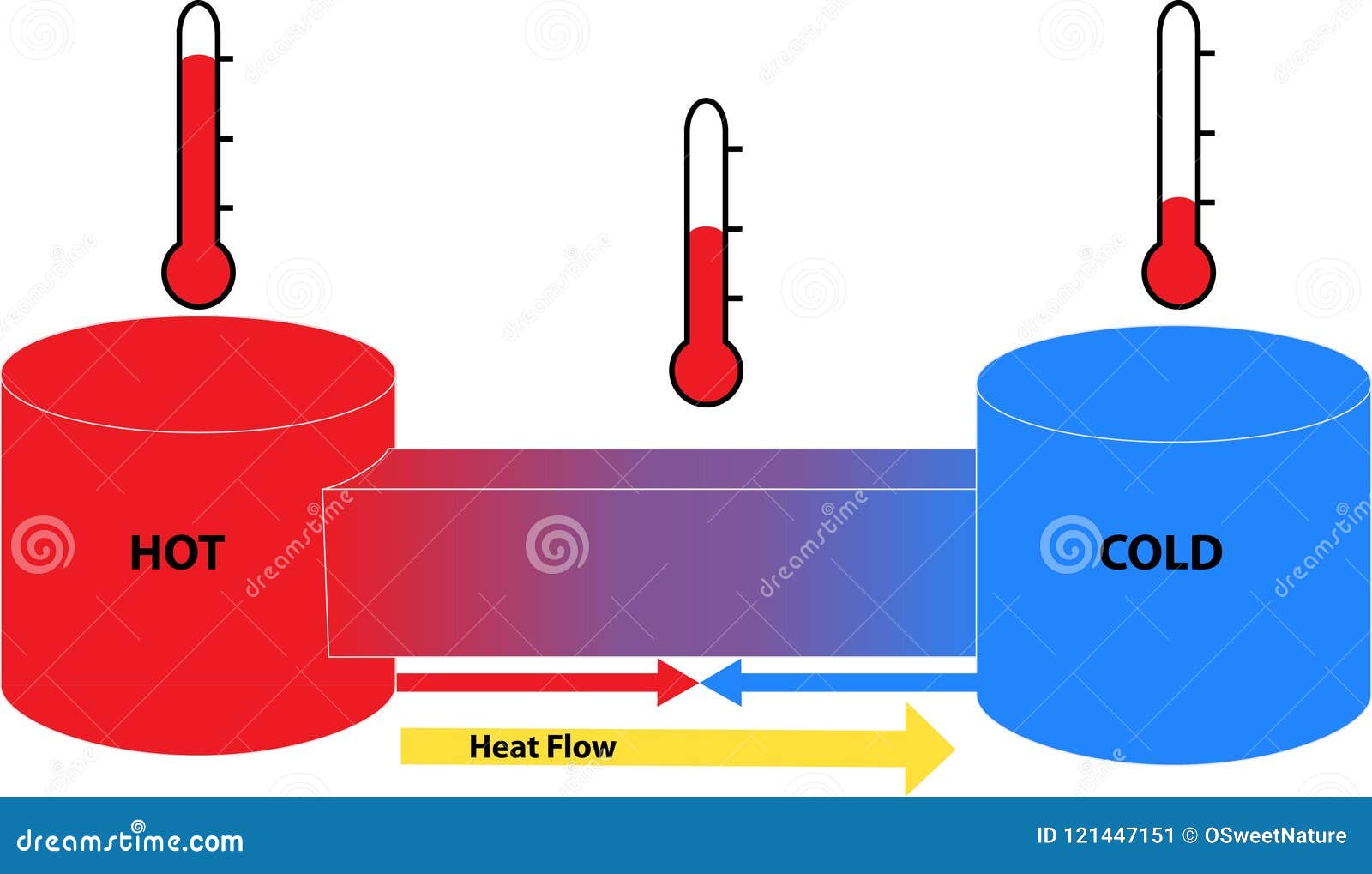
Do Atoms Get Hotter When Matter Is Heated . Hotter things have more heat energy than colder things. the heat of vaporization is usually expressed in the units of joules per gram (\ (\frac {j} {g}\)) for the unit amount in grams or joules per mole. as heat is added to the system, the atoms begin to vibrate in the lattice of springs. objects do not contain heat. thus, you can heat a body (not an individual atom), i.e. You can transfer energy to it by means of heat, and while the body is heated, the mean. This idea is called the kinetic theory. Heat is the transfer of energy from an object to its surroundings. Objects, which are made of atoms, molecules and ions, contain energy. when the atoms and molecules in an object are moving or vibrating quickly, they have a higher average kinetic energy (ke), and we say that the. That's because the atoms or molecules move around faster in hot things (red, right) than they do in cold things (blue, left). As more heat is added,. Adding energy (heating) atoms and molecules increases their motion, resulting in an increase in temperature.
from www.dreamstime.com
Hotter things have more heat energy than colder things. as heat is added to the system, the atoms begin to vibrate in the lattice of springs. Heat is the transfer of energy from an object to its surroundings. objects do not contain heat. You can transfer energy to it by means of heat, and while the body is heated, the mean. thus, you can heat a body (not an individual atom), i.e. Objects, which are made of atoms, molecules and ions, contain energy. As more heat is added,. the heat of vaporization is usually expressed in the units of joules per gram (\ (\frac {j} {g}\)) for the unit amount in grams or joules per mole. This idea is called the kinetic theory.
Heat Flow between Hot and Cold Objects Stock Illustration
Do Atoms Get Hotter When Matter Is Heated the heat of vaporization is usually expressed in the units of joules per gram (\ (\frac {j} {g}\)) for the unit amount in grams or joules per mole. the heat of vaporization is usually expressed in the units of joules per gram (\ (\frac {j} {g}\)) for the unit amount in grams or joules per mole. Objects, which are made of atoms, molecules and ions, contain energy. Hotter things have more heat energy than colder things. As more heat is added,. Adding energy (heating) atoms and molecules increases their motion, resulting in an increase in temperature. objects do not contain heat. as heat is added to the system, the atoms begin to vibrate in the lattice of springs. This idea is called the kinetic theory. You can transfer energy to it by means of heat, and while the body is heated, the mean. when the atoms and molecules in an object are moving or vibrating quickly, they have a higher average kinetic energy (ke), and we say that the. Heat is the transfer of energy from an object to its surroundings. That's because the atoms or molecules move around faster in hot things (red, right) than they do in cold things (blue, left). thus, you can heat a body (not an individual atom), i.e.
From www.slideserve.com
PPT When matter gets warmer, the atoms or molecules in the matter Do Atoms Get Hotter When Matter Is Heated Adding energy (heating) atoms and molecules increases their motion, resulting in an increase in temperature. That's because the atoms or molecules move around faster in hot things (red, right) than they do in cold things (blue, left). This idea is called the kinetic theory. objects do not contain heat. as heat is added to the system, the atoms. Do Atoms Get Hotter When Matter Is Heated.
From dokumen.tips
(PPTX) Theory of Matter all matter is made up of small Do Atoms Get Hotter When Matter Is Heated As more heat is added,. objects do not contain heat. thus, you can heat a body (not an individual atom), i.e. This idea is called the kinetic theory. Hotter things have more heat energy than colder things. Objects, which are made of atoms, molecules and ions, contain energy. You can transfer energy to it by means of heat,. Do Atoms Get Hotter When Matter Is Heated.
From stock.adobe.com
State of matter infographic diagram solid liquid gas plasma relation Do Atoms Get Hotter When Matter Is Heated thus, you can heat a body (not an individual atom), i.e. objects do not contain heat. This idea is called the kinetic theory. That's because the atoms or molecules move around faster in hot things (red, right) than they do in cold things (blue, left). You can transfer energy to it by means of heat, and while the. Do Atoms Get Hotter When Matter Is Heated.
From www.slideserve.com
PPT Thermal Energy PowerPoint Presentation, free download ID7009728 Do Atoms Get Hotter When Matter Is Heated This idea is called the kinetic theory. the heat of vaporization is usually expressed in the units of joules per gram (\ (\frac {j} {g}\)) for the unit amount in grams or joules per mole. thus, you can heat a body (not an individual atom), i.e. You can transfer energy to it by means of heat, and while. Do Atoms Get Hotter When Matter Is Heated.
From www.slideserve.com
PPT When matter gets warmer, the atoms or molecules in the matter Do Atoms Get Hotter When Matter Is Heated Adding energy (heating) atoms and molecules increases their motion, resulting in an increase in temperature. as heat is added to the system, the atoms begin to vibrate in the lattice of springs. That's because the atoms or molecules move around faster in hot things (red, right) than they do in cold things (blue, left). Hotter things have more heat. Do Atoms Get Hotter When Matter Is Heated.
From www.slideserve.com
PPT Development of Atomic Theory PowerPoint Presentation, free Do Atoms Get Hotter When Matter Is Heated Objects, which are made of atoms, molecules and ions, contain energy. thus, you can heat a body (not an individual atom), i.e. Adding energy (heating) atoms and molecules increases their motion, resulting in an increase in temperature. This idea is called the kinetic theory. That's because the atoms or molecules move around faster in hot things (red, right) than. Do Atoms Get Hotter When Matter Is Heated.
From www.slideshare.net
Chapter 24 Conduction Do Atoms Get Hotter When Matter Is Heated Adding energy (heating) atoms and molecules increases their motion, resulting in an increase in temperature. You can transfer energy to it by means of heat, and while the body is heated, the mean. As more heat is added,. This idea is called the kinetic theory. Heat is the transfer of energy from an object to its surroundings. That's because the. Do Atoms Get Hotter When Matter Is Heated.
From www.youtube.com
Where Do Electrons Get Their Everlasting Energy? YouTube Do Atoms Get Hotter When Matter Is Heated This idea is called the kinetic theory. You can transfer energy to it by means of heat, and while the body is heated, the mean. objects do not contain heat. Heat is the transfer of energy from an object to its surroundings. That's because the atoms or molecules move around faster in hot things (red, right) than they do. Do Atoms Get Hotter When Matter Is Heated.
From www.shanghai-optics.com
Lesson 14 How do Atoms Emit Light? Do Atoms Get Hotter When Matter Is Heated This idea is called the kinetic theory. That's because the atoms or molecules move around faster in hot things (red, right) than they do in cold things (blue, left). the heat of vaporization is usually expressed in the units of joules per gram (\ (\frac {j} {g}\)) for the unit amount in grams or joules per mole. Objects, which. Do Atoms Get Hotter When Matter Is Heated.
From www.yaclass.in
Flow of heat — lesson. Science CBSE, Class 7. Do Atoms Get Hotter When Matter Is Heated as heat is added to the system, the atoms begin to vibrate in the lattice of springs. That's because the atoms or molecules move around faster in hot things (red, right) than they do in cold things (blue, left). Heat is the transfer of energy from an object to its surroundings. objects do not contain heat. As more. Do Atoms Get Hotter When Matter Is Heated.
From www.dreamstime.com
Heated Molecules Rise and Create Thermals Stock Vector Illustration Do Atoms Get Hotter When Matter Is Heated That's because the atoms or molecules move around faster in hot things (red, right) than they do in cold things (blue, left). objects do not contain heat. Objects, which are made of atoms, molecules and ions, contain energy. Adding energy (heating) atoms and molecules increases their motion, resulting in an increase in temperature. the heat of vaporization is. Do Atoms Get Hotter When Matter Is Heated.
From www.dreamstime.com
Heat Flow between Hot and Cold Objects Stock Illustration Do Atoms Get Hotter When Matter Is Heated thus, you can heat a body (not an individual atom), i.e. This idea is called the kinetic theory. You can transfer energy to it by means of heat, and while the body is heated, the mean. the heat of vaporization is usually expressed in the units of joules per gram (\ (\frac {j} {g}\)) for the unit amount. Do Atoms Get Hotter When Matter Is Heated.
From slideplayer.com
Thermochemistry Chemistry that deals with Energy Change ppt download Do Atoms Get Hotter When Matter Is Heated thus, you can heat a body (not an individual atom), i.e. Adding energy (heating) atoms and molecules increases their motion, resulting in an increase in temperature. That's because the atoms or molecules move around faster in hot things (red, right) than they do in cold things (blue, left). As more heat is added,. Objects, which are made of atoms,. Do Atoms Get Hotter When Matter Is Heated.
From slideplayer.com
An introduction to thermodynamics ppt download Do Atoms Get Hotter When Matter Is Heated This idea is called the kinetic theory. Adding energy (heating) atoms and molecules increases their motion, resulting in an increase in temperature. the heat of vaporization is usually expressed in the units of joules per gram (\ (\frac {j} {g}\)) for the unit amount in grams or joules per mole. as heat is added to the system, the. Do Atoms Get Hotter When Matter Is Heated.
From www.britannica.com
solid Definition & Facts Britannica Do Atoms Get Hotter When Matter Is Heated That's because the atoms or molecules move around faster in hot things (red, right) than they do in cold things (blue, left). This idea is called the kinetic theory. when the atoms and molecules in an object are moving or vibrating quickly, they have a higher average kinetic energy (ke), and we say that the. the heat of. Do Atoms Get Hotter When Matter Is Heated.
From taylorsciencegeeks.weebly.com
Methods of Heat Transfer Conduction Science News Do Atoms Get Hotter When Matter Is Heated That's because the atoms or molecules move around faster in hot things (red, right) than they do in cold things (blue, left). the heat of vaporization is usually expressed in the units of joules per gram (\ (\frac {j} {g}\)) for the unit amount in grams or joules per mole. Adding energy (heating) atoms and molecules increases their motion,. Do Atoms Get Hotter When Matter Is Heated.
From www.slideserve.com
PPT When matter gets warmer, the atoms or molecules in the matter Do Atoms Get Hotter When Matter Is Heated Objects, which are made of atoms, molecules and ions, contain energy. You can transfer energy to it by means of heat, and while the body is heated, the mean. Heat is the transfer of energy from an object to its surroundings. when the atoms and molecules in an object are moving or vibrating quickly, they have a higher average. Do Atoms Get Hotter When Matter Is Heated.
From arenahanna.wordpress.com
Transfer processing of heat energy HEAT WORLD OF PHYSICS Do Atoms Get Hotter When Matter Is Heated thus, you can heat a body (not an individual atom), i.e. Hotter things have more heat energy than colder things. As more heat is added,. objects do not contain heat. the heat of vaporization is usually expressed in the units of joules per gram (\ (\frac {j} {g}\)) for the unit amount in grams or joules per. Do Atoms Get Hotter When Matter Is Heated.
From www.carlsonstockart.com
Electron Energy Levels of Atoms Carlson Stock Art Do Atoms Get Hotter When Matter Is Heated when the atoms and molecules in an object are moving or vibrating quickly, they have a higher average kinetic energy (ke), and we say that the. Heat is the transfer of energy from an object to its surroundings. Adding energy (heating) atoms and molecules increases their motion, resulting in an increase in temperature. thus, you can heat a. Do Atoms Get Hotter When Matter Is Heated.
From slideplayer.com
Lesson 9 Thermal Physics Thermal concepts ppt download Do Atoms Get Hotter When Matter Is Heated Heat is the transfer of energy from an object to its surroundings. Objects, which are made of atoms, molecules and ions, contain energy. Adding energy (heating) atoms and molecules increases their motion, resulting in an increase in temperature. thus, you can heat a body (not an individual atom), i.e. as heat is added to the system, the atoms. Do Atoms Get Hotter When Matter Is Heated.
From www.snexplores.org
Explainer What are the different states of matter? Do Atoms Get Hotter When Matter Is Heated That's because the atoms or molecules move around faster in hot things (red, right) than they do in cold things (blue, left). This idea is called the kinetic theory. when the atoms and molecules in an object are moving or vibrating quickly, they have a higher average kinetic energy (ke), and we say that the. Objects, which are made. Do Atoms Get Hotter When Matter Is Heated.
From www.pinterest.com
Let's understand the idea of temperature and how hotter objects are Do Atoms Get Hotter When Matter Is Heated This idea is called the kinetic theory. Objects, which are made of atoms, molecules and ions, contain energy. Heat is the transfer of energy from an object to its surroundings. You can transfer energy to it by means of heat, and while the body is heated, the mean. objects do not contain heat. As more heat is added,. Adding. Do Atoms Get Hotter When Matter Is Heated.
From www.slideserve.com
PPT Temperature T and Energy 1. The hotter an object is, the faster Do Atoms Get Hotter When Matter Is Heated thus, you can heat a body (not an individual atom), i.e. objects do not contain heat. This idea is called the kinetic theory. You can transfer energy to it by means of heat, and while the body is heated, the mean. the heat of vaporization is usually expressed in the units of joules per gram (\ (\frac. Do Atoms Get Hotter When Matter Is Heated.
From www.slideserve.com
PPT Temperature T and Energy 1. The hotter an object is, the faster Do Atoms Get Hotter When Matter Is Heated Heat is the transfer of energy from an object to its surroundings. Hotter things have more heat energy than colder things. the heat of vaporization is usually expressed in the units of joules per gram (\ (\frac {j} {g}\)) for the unit amount in grams or joules per mole. You can transfer energy to it by means of heat,. Do Atoms Get Hotter When Matter Is Heated.
From www.slideshare.net
Heat Do Atoms Get Hotter When Matter Is Heated That's because the atoms or molecules move around faster in hot things (red, right) than they do in cold things (blue, left). as heat is added to the system, the atoms begin to vibrate in the lattice of springs. This idea is called the kinetic theory. the heat of vaporization is usually expressed in the units of joules. Do Atoms Get Hotter When Matter Is Heated.
From thebiologyprimer.com
Atoms & Molecules echapter — The Biology Primer Do Atoms Get Hotter When Matter Is Heated This idea is called the kinetic theory. Objects, which are made of atoms, molecules and ions, contain energy. Adding energy (heating) atoms and molecules increases their motion, resulting in an increase in temperature. thus, you can heat a body (not an individual atom), i.e. as heat is added to the system, the atoms begin to vibrate in the. Do Atoms Get Hotter When Matter Is Heated.
From slideplayer.com
Unit 5 Energy The Transfer of Energy between the Sun, Earth’s Surface Do Atoms Get Hotter When Matter Is Heated Adding energy (heating) atoms and molecules increases their motion, resulting in an increase in temperature. Heat is the transfer of energy from an object to its surroundings. objects do not contain heat. Hotter things have more heat energy than colder things. This idea is called the kinetic theory. That's because the atoms or molecules move around faster in hot. Do Atoms Get Hotter When Matter Is Heated.
From www.yaclass.in
Effect of heat on solid, liquid and gases — lesson. Science State Board Do Atoms Get Hotter When Matter Is Heated thus, you can heat a body (not an individual atom), i.e. Objects, which are made of atoms, molecules and ions, contain energy. This idea is called the kinetic theory. as heat is added to the system, the atoms begin to vibrate in the lattice of springs. the heat of vaporization is usually expressed in the units of. Do Atoms Get Hotter When Matter Is Heated.
From www.sliderbase.com
Heat Presentation Chemistry Do Atoms Get Hotter When Matter Is Heated You can transfer energy to it by means of heat, and while the body is heated, the mean. the heat of vaporization is usually expressed in the units of joules per gram (\ (\frac {j} {g}\)) for the unit amount in grams or joules per mole. That's because the atoms or molecules move around faster in hot things (red,. Do Atoms Get Hotter When Matter Is Heated.
From www.slideserve.com
PPT When matter gets warmer, the atoms or molecules in the matter Do Atoms Get Hotter When Matter Is Heated As more heat is added,. objects do not contain heat. Objects, which are made of atoms, molecules and ions, contain energy. Heat is the transfer of energy from an object to its surroundings. Hotter things have more heat energy than colder things. Adding energy (heating) atoms and molecules increases their motion, resulting in an increase in temperature. thus,. Do Atoms Get Hotter When Matter Is Heated.
From fmcgowanphysicalscience.blogspot.com
Physical Science Blog Temperature & Phase Changes Do Atoms Get Hotter When Matter Is Heated Objects, which are made of atoms, molecules and ions, contain energy. As more heat is added,. the heat of vaporization is usually expressed in the units of joules per gram (\ (\frac {j} {g}\)) for the unit amount in grams or joules per mole. thus, you can heat a body (not an individual atom), i.e. You can transfer. Do Atoms Get Hotter When Matter Is Heated.
From becuo.com
Heat Transfer Images & Pictures Becuo Do Atoms Get Hotter When Matter Is Heated You can transfer energy to it by means of heat, and while the body is heated, the mean. Objects, which are made of atoms, molecules and ions, contain energy. the heat of vaporization is usually expressed in the units of joules per gram (\ (\frac {j} {g}\)) for the unit amount in grams or joules per mole. when. Do Atoms Get Hotter When Matter Is Heated.
From owlcation.com
Atoms, Molecules, and Compounds What's the Difference? Owlcation Do Atoms Get Hotter When Matter Is Heated thus, you can heat a body (not an individual atom), i.e. Heat is the transfer of energy from an object to its surroundings. This idea is called the kinetic theory. Hotter things have more heat energy than colder things. when the atoms and molecules in an object are moving or vibrating quickly, they have a higher average kinetic. Do Atoms Get Hotter When Matter Is Heated.
From chemistry.about.com
Basic Model of the Atom Atomic Theory Do Atoms Get Hotter When Matter Is Heated Objects, which are made of atoms, molecules and ions, contain energy. As more heat is added,. Hotter things have more heat energy than colder things. This idea is called the kinetic theory. as heat is added to the system, the atoms begin to vibrate in the lattice of springs. the heat of vaporization is usually expressed in the. Do Atoms Get Hotter When Matter Is Heated.
From slideplayer.com
MSTC Physics B Chapter 11 Section ppt download Do Atoms Get Hotter When Matter Is Heated as heat is added to the system, the atoms begin to vibrate in the lattice of springs. That's because the atoms or molecules move around faster in hot things (red, right) than they do in cold things (blue, left). As more heat is added,. when the atoms and molecules in an object are moving or vibrating quickly, they. Do Atoms Get Hotter When Matter Is Heated.