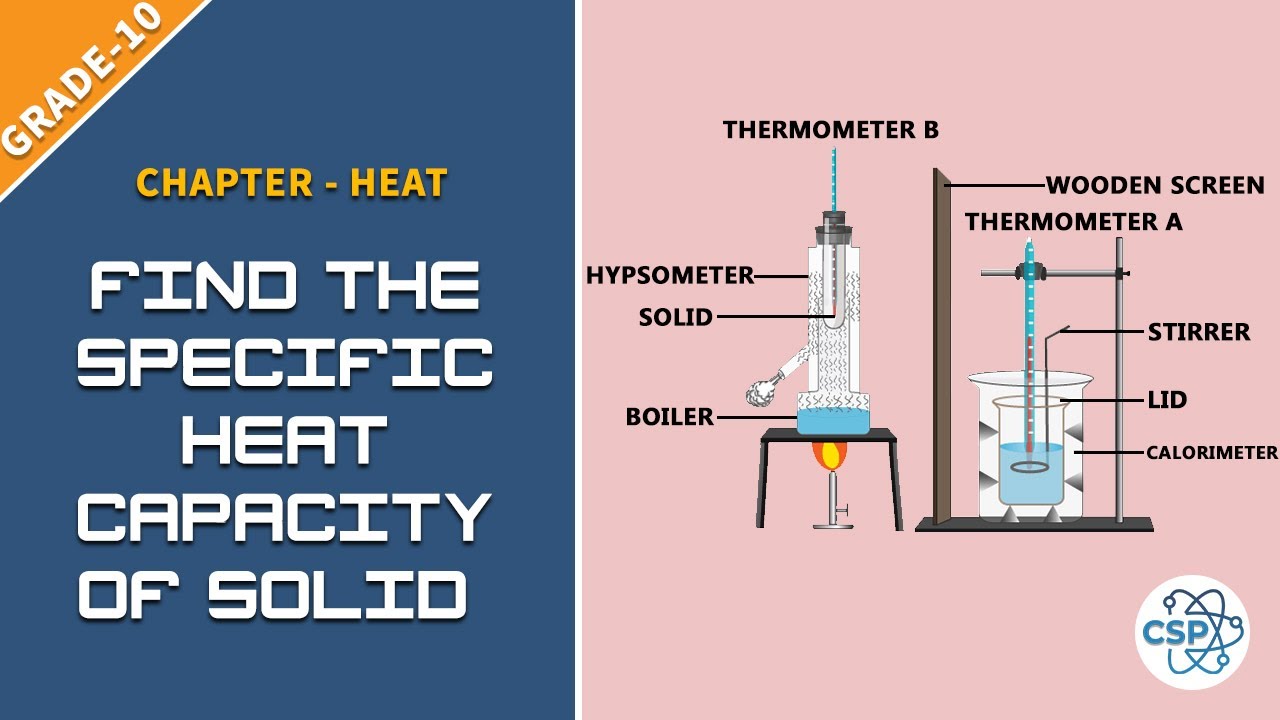
How Much Fuel Is In A Calorimeter . Find out how to calculate the energy per gram of fuel and the factors affecting the accuracy of the experiment. For example, when an exothermic. Learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Learn how to measure heat energy released by burning fuels using calorimetry. The vessel is filled with water, and the fuel is burned, leading to the heating of the water. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of. Find the specific heat, molar heat. Heat loss by the fuel is equal to the heat. Say in a calorimeter a fixed amount of fuel is burned. The amount of energy given out by burning a fuel in a calorimeter can be calculated using. How much energy is released from burning a fuel? Learn how to measure and calculate heat and enthalpy changes using calorimetry techniques.
from porter-yersblogvega.blogspot.com
For example, when an exothermic. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of. The amount of energy given out by burning a fuel in a calorimeter can be calculated using. Find the specific heat, molar heat. Say in a calorimeter a fixed amount of fuel is burned. Learn how to measure heat energy released by burning fuels using calorimetry. Find out how to calculate the energy per gram of fuel and the factors affecting the accuracy of the experiment. How much energy is released from burning a fuel? Learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. Heat loss by the fuel is equal to the heat.
Heat Capacity of Calorimeter
How Much Fuel Is In A Calorimeter Find the specific heat, molar heat. Find out how to calculate the energy per gram of fuel and the factors affecting the accuracy of the experiment. Heat loss by the fuel is equal to the heat. Say in a calorimeter a fixed amount of fuel is burned. The amount of energy given out by burning a fuel in a calorimeter can be calculated using. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. How much energy is released from burning a fuel? Find the specific heat, molar heat. Learn how to measure heat energy released by burning fuels using calorimetry. For example, when an exothermic. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of. The vessel is filled with water, and the fuel is burned, leading to the heating of the water. Learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. Learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions.
From studylib.net
GasCVD® Natural Gas Calorimeter Model CVM400 How Much Fuel Is In A Calorimeter Find the specific heat, molar heat. Learn how to measure heat energy released by burning fuels using calorimetry. For example, when an exothermic. Learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of. The amount of. How Much Fuel Is In A Calorimeter.
From www.astrascientific.com
Gas and Bomb Calorimeter Manufacturers, Gas and Bomb How Much Fuel Is In A Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The amount of energy given out by burning a fuel in a calorimeter can be calculated using. For example, when an exothermic. Say in a calorimeter a fixed amount of fuel is burned. How much energy is released from burning a. How Much Fuel Is In A Calorimeter.
From www.youtube.com
Junkers Gas Calorimeter Experiment Boys Gas Calorimeter Experiment How Much Fuel Is In A Calorimeter Find out how to calculate the energy per gram of fuel and the factors affecting the accuracy of the experiment. The amount of energy given out by burning a fuel in a calorimeter can be calculated using. Learn how to measure heat energy released by burning fuels using calorimetry. Say in a calorimeter a fixed amount of fuel is burned.. How Much Fuel Is In A Calorimeter.
From www.gbu-presnenskij.ru
Chapter 25 Essential Calorimetry Formulae James Kennedy, 47 OFF How Much Fuel Is In A Calorimeter Learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. Heat loss by the fuel is equal to the heat. Learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. The amount of energy given out by burning a fuel in a calorimeter can be calculated using. The vessel is. How Much Fuel Is In A Calorimeter.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry How Much Fuel Is In A Calorimeter Learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. Find the specific heat, molar heat. The vessel is filled with water, and the fuel is burned, leading to the heating of the water. Say in a calorimeter a fixed amount of fuel is burned. Heat loss by the fuel is equal to. How Much Fuel Is In A Calorimeter.
From porter-yersblogvega.blogspot.com
Heat Capacity of Calorimeter How Much Fuel Is In A Calorimeter How much energy is released from burning a fuel? A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. Say in a calorimeter a fixed amount of fuel is burned. Learn how to measure heat energy released by. How Much Fuel Is In A Calorimeter.
From www.baamboozle.com
Heat Gain and Heat Loss Baamboozle Baamboozle The Most Fun How Much Fuel Is In A Calorimeter The amount of energy given out by burning a fuel in a calorimeter can be calculated using. The vessel is filled with water, and the fuel is burned, leading to the heating of the water. Learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. Find the specific heat, molar heat. Heat loss by the fuel is. How Much Fuel Is In A Calorimeter.
From rattibha.com
السعرات الحرارية للغذاء.. كيف بدأت وفين وصلت.. وكيف ممكن تستفيد لما How Much Fuel Is In A Calorimeter The vessel is filled with water, and the fuel is burned, leading to the heating of the water. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Learn how to measure heat energy released by burning fuels using calorimetry. How much energy is released from. How Much Fuel Is In A Calorimeter.
From haipernews.com
How To Calculate How Many Joules Of Heat Haiper How Much Fuel Is In A Calorimeter How much energy is released from burning a fuel? For example, when an exothermic. The amount of energy given out by burning a fuel in a calorimeter can be calculated using. Learn how to measure heat energy released by burning fuels using calorimetry. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or. How Much Fuel Is In A Calorimeter.
From www.scribd.com
Junker Gas Calorimeter PDF Gases Temperature How Much Fuel Is In A Calorimeter Heat loss by the fuel is equal to the heat. Learn how to measure heat energy released by burning fuels using calorimetry. Find the specific heat, molar heat. The amount of energy given out by burning a fuel in a calorimeter can be calculated using. Find out how to calculate the energy per gram of fuel and the factors affecting. How Much Fuel Is In A Calorimeter.
From giovlaesa.blob.core.windows.net
How Much Fuel Does A Freighter Use at Amanda Nagy blog How Much Fuel Is In A Calorimeter For example, when an exothermic. Say in a calorimeter a fixed amount of fuel is burned. Learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. Heat loss by the fuel is equal to the heat. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or. How Much Fuel Is In A Calorimeter.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii How Much Fuel Is In A Calorimeter How much energy is released from burning a fuel? For example, when an exothermic. The amount of energy given out by burning a fuel in a calorimeter can be calculated using. Find out how to calculate the energy per gram of fuel and the factors affecting the accuracy of the experiment. Learn how to measure heat energy released by burning. How Much Fuel Is In A Calorimeter.
From www.toppr.com
In a constant volume calorimeter, 3.5 g of a gas with molecular weight How Much Fuel Is In A Calorimeter Learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. The vessel is filled with water, and the fuel is burned, leading to the heating of the water. Learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. Say in a calorimeter a fixed amount of fuel is burned. Find. How Much Fuel Is In A Calorimeter.
From slideplayer.com
A “Calorimeter”. Calorimetry Calculations When analyzing data obtained How Much Fuel Is In A Calorimeter Find the specific heat, molar heat. Learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. Learn how to measure heat energy released by burning fuels using calorimetry. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of. Say in a calorimeter a fixed amount of. How Much Fuel Is In A Calorimeter.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation How Much Fuel Is In A Calorimeter Learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. The vessel is filled with water, and the fuel is burned, leading to the heating of the water. Find the specific heat, molar heat. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. How Much Fuel Is In A Calorimeter.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson How Much Fuel Is In A Calorimeter Learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. Find out how to calculate the energy per gram of fuel and the factors affecting the accuracy of the experiment. For example, when an exothermic. Learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. Calorimetry is an application of. How Much Fuel Is In A Calorimeter.
From www.numerade.com
SOLVED A peanut was burned in a calorimeter and the following data was How Much Fuel Is In A Calorimeter The amount of energy given out by burning a fuel in a calorimeter can be calculated using. Learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. How much energy is released from burning a fuel? Find out how to calculate the energy per gram of fuel and the factors affecting the accuracy. How Much Fuel Is In A Calorimeter.
From www.pinterest.com.au
Pin on Science Friday How Much Fuel Is In A Calorimeter Heat loss by the fuel is equal to the heat. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of. How much energy is released from burning a fuel? Find the specific heat, molar heat. The vessel is filled with water, and the fuel is burned, leading to the. How Much Fuel Is In A Calorimeter.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment How Much Fuel Is In A Calorimeter Say in a calorimeter a fixed amount of fuel is burned. For example, when an exothermic. The amount of energy given out by burning a fuel in a calorimeter can be calculated using. Learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. How much energy is released from burning a fuel? Learn. How Much Fuel Is In A Calorimeter.
From courses.lumenlearning.com
Calorimetry CHEM 1305 General Chemistry I—Lecture How Much Fuel Is In A Calorimeter Say in a calorimeter a fixed amount of fuel is burned. Learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Heat loss by the fuel is equal to the heat. Learn how to. How Much Fuel Is In A Calorimeter.
From engineeringlearn.com
Bomb Calorimeter Definition, Construction, Diagram, Working & Uses How Much Fuel Is In A Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Heat loss by the fuel is equal to the heat. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of. Learn how to calculate and interpret heat capacity, enthalpy, and. How Much Fuel Is In A Calorimeter.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry How Much Fuel Is In A Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Find the specific heat, molar heat. The vessel is filled with water, and the fuel is burned, leading to the heating of the water. For example, when an exothermic. Say in a calorimeter a fixed amount of fuel is burned. The. How Much Fuel Is In A Calorimeter.
From www.numerade.com
SOLVED The following data were obtained in a Boy's gas calorimeter How Much Fuel Is In A Calorimeter Find out how to calculate the energy per gram of fuel and the factors affecting the accuracy of the experiment. Learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. The vessel is filled with water, and the fuel is burned, leading to the heating of the water. Learn how to measure and. How Much Fuel Is In A Calorimeter.
From www.youtube.com
Principle of Calorimetry YouTube How Much Fuel Is In A Calorimeter Find the specific heat, molar heat. Find out how to calculate the energy per gram of fuel and the factors affecting the accuracy of the experiment. Learn how to measure heat energy released by burning fuels using calorimetry. For example, when an exothermic. How much energy is released from burning a fuel? The amount of energy given out by burning. How Much Fuel Is In A Calorimeter.
From socratic.org
Question 5d3f9 + Example How Much Fuel Is In A Calorimeter A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The vessel is filled with water, and the fuel is burned, leading to the heating of the water. Learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. How much energy is released from burning a fuel? Calorimetry. How Much Fuel Is In A Calorimeter.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle How Much Fuel Is In A Calorimeter How much energy is released from burning a fuel? A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. Find the specific heat, molar heat. Learn how to measure and calculate heat and enthalpy. How Much Fuel Is In A Calorimeter.
From studylib.net
Heat Capacity of Metals PreLab How Much Fuel Is In A Calorimeter Heat loss by the fuel is equal to the heat. The vessel is filled with water, and the fuel is burned, leading to the heating of the water. How much energy is released from burning a fuel? The amount of energy given out by burning a fuel in a calorimeter can be calculated using. Learn how to measure heat energy. How Much Fuel Is In A Calorimeter.
From www.scribd.com
Calorific Value of Fuel Using Junker S Gas Calorimeter PDF Applied How Much Fuel Is In A Calorimeter Learn how to calculate and interpret heat capacity, enthalpy, and calorimetry data for different substances and reactions. Find out how to calculate the energy per gram of fuel and the factors affecting the accuracy of the experiment. For example, when an exothermic. The vessel is filled with water, and the fuel is burned, leading to the heating of the water.. How Much Fuel Is In A Calorimeter.
From www.youtube.com
How to find calorific value of fuel? Construction and working of Bomb How Much Fuel Is In A Calorimeter Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of. The vessel is filled with water, and the fuel is burned, leading to the heating of the water. For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical. How Much Fuel Is In A Calorimeter.
From www.linstitute.net
IB DP Chemistry HL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 How Much Fuel Is In A Calorimeter Learn how to measure heat energy released by burning fuels using calorimetry. The vessel is filled with water, and the fuel is burned, leading to the heating of the water. How much energy is released from burning a fuel? Find out how to calculate the energy per gram of fuel and the factors affecting the accuracy of the experiment. Heat. How Much Fuel Is In A Calorimeter.
From www.bartleby.com
Answered A bomb calorimeter, or a constant… bartleby How Much Fuel Is In A Calorimeter Heat loss by the fuel is equal to the heat. How much energy is released from burning a fuel? A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. For example, when an exothermic. Learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. Learn how to calculate. How Much Fuel Is In A Calorimeter.
From www.pinterest.com
a speedometer with the text how much fuel is in the reserve tank How Much Fuel Is In A Calorimeter Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of. Say in a calorimeter a fixed amount of fuel is burned. Find out how to calculate the energy per gram of fuel and the factors affecting the accuracy of the experiment. Learn how to measure heat energy released by. How Much Fuel Is In A Calorimeter.
From www.chegg.com
Use the interactive to observe the temperature change How Much Fuel Is In A Calorimeter For example, when an exothermic. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The amount of energy given out by burning a fuel in a calorimeter can be calculated using. Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat. How Much Fuel Is In A Calorimeter.
From www.numerade.com
SOLVED Describe how the calorific value of a solid is determined using How Much Fuel Is In A Calorimeter Find the specific heat, molar heat. Learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. Say in a calorimeter a fixed amount of fuel is burned. The vessel is filled with water, and the fuel is burned, leading to the heating of the water. The amount of energy given out by burning a fuel in a. How Much Fuel Is In A Calorimeter.
From www.indiamart.com
Junker''S Gas Calorimeter, Model Number BSC399, Model BSC339, Rs How Much Fuel Is In A Calorimeter Calorimetry is an application of the first law of thermodynamics to measure the enthalpies of reaction or the heat capacities of. The vessel is filled with water, and the fuel is burned, leading to the heating of the water. Learn how to measure and calculate heat and enthalpy changes using calorimetry techniques. Learn how to measure heat energy released by. How Much Fuel Is In A Calorimeter.