Ideal Gas In Physics . See examples of how to use the ideal gas law to calculate changes. Learn how the ideal gas law relates the pressure, volume, temperature, and number of atoms or molecules of a gas. Learn about the ideal gas law, a general equation of state that relates the pressure, volume, and temperature of a gas. Explore the properties and processes of ideal gases, such. Learn how the ideal gas law (pv=nrt or pv=nkt) is derived from empirical and microscopic considerations. Find out how to use the universal gas constant r and the kinetic. An ideal gas is a gas that obeys the equation \\ (pv = nkt\\), where \\ (p\\) is pressure, \\ (v\\) is volume, \\ (n\\) is number of molecules, and \\ (t\\) is temperature. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states.
from www.slideserve.com
Find out how to use the universal gas constant r and the kinetic. An ideal gas is a gas that obeys the equation \\ (pv = nkt\\), where \\ (p\\) is pressure, \\ (v\\) is volume, \\ (n\\) is number of molecules, and \\ (t\\) is temperature. See examples of how to use the ideal gas law to calculate changes. Learn how the ideal gas law relates the pressure, volume, temperature, and number of atoms or molecules of a gas. Explore the properties and processes of ideal gases, such. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Learn about the ideal gas law, a general equation of state that relates the pressure, volume, and temperature of a gas. Learn how the ideal gas law (pv=nrt or pv=nkt) is derived from empirical and microscopic considerations.
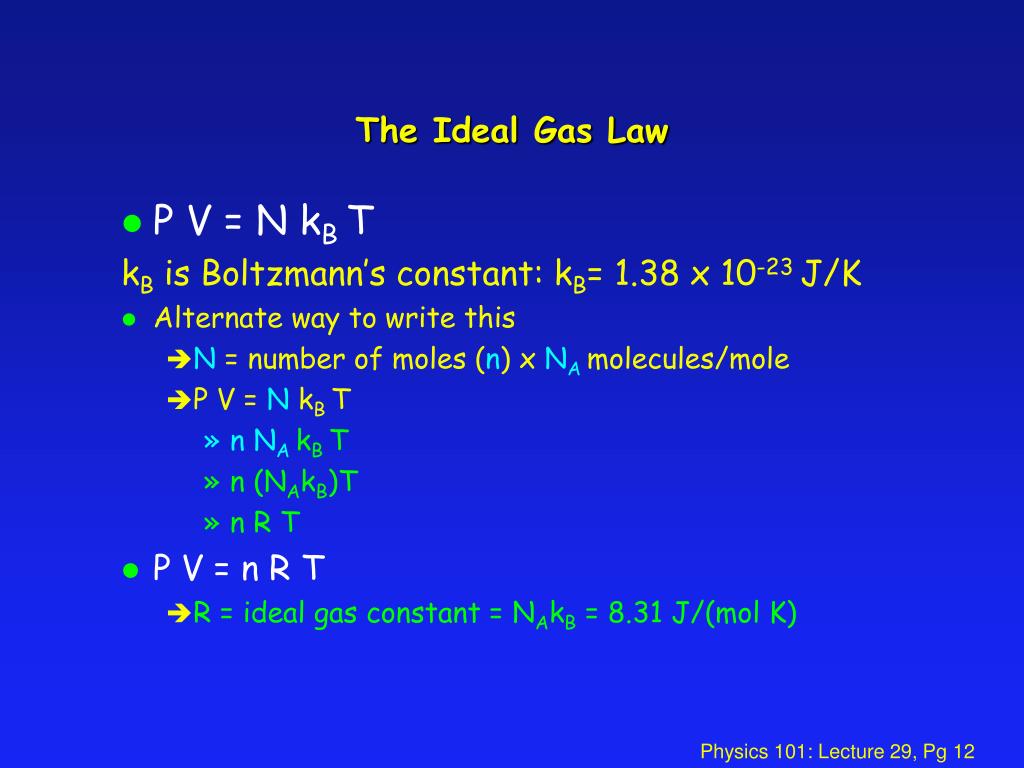
PPT Physics 101 Lecture 29 Ideal Gas Law & Theory PowerPoint
Ideal Gas In Physics See examples of how to use the ideal gas law to calculate changes. Find out how to use the universal gas constant r and the kinetic. Learn how the ideal gas law (pv=nrt or pv=nkt) is derived from empirical and microscopic considerations. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Explore the properties and processes of ideal gases, such. See examples of how to use the ideal gas law to calculate changes. Learn how the ideal gas law relates the pressure, volume, temperature, and number of atoms or molecules of a gas. Learn about the ideal gas law, a general equation of state that relates the pressure, volume, and temperature of a gas. An ideal gas is a gas that obeys the equation \\ (pv = nkt\\), where \\ (p\\) is pressure, \\ (v\\) is volume, \\ (n\\) is number of molecules, and \\ (t\\) is temperature.
From www.alamy.com
Ideal gas law formula. Pressure, volume, amount of substance , ideal Ideal Gas In Physics Find out how to use the universal gas constant r and the kinetic. Learn how the ideal gas law relates the pressure, volume, temperature, and number of atoms or molecules of a gas. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. An. Ideal Gas In Physics.
From sciencenotes.org
Ideal Gas Law Formula and Examples Ideal Gas In Physics Learn about the ideal gas law, a general equation of state that relates the pressure, volume, and temperature of a gas. Learn how the ideal gas law (pv=nrt or pv=nkt) is derived from empirical and microscopic considerations. See examples of how to use the ideal gas law to calculate changes. Find out how to use the universal gas constant r. Ideal Gas In Physics.
From www.youtube.com
Ideal Gas Assumptions Theory YouTube Ideal Gas In Physics Learn how the ideal gas law relates the pressure, volume, temperature, and number of atoms or molecules of a gas. An ideal gas is a gas that obeys the equation \\ (pv = nkt\\), where \\ (p\\) is pressure, \\ (v\\) is volume, \\ (n\\) is number of molecules, and \\ (t\\) is temperature. Ideal gas, a gas that conforms,. Ideal Gas In Physics.
From owlcation.com
The Theories and Behavior of Gas Owlcation Ideal Gas In Physics Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Find out how to use the universal gas constant r and the kinetic. Explore the properties and processes of ideal gases, such. Learn how the ideal gas law relates the pressure, volume, temperature, and. Ideal Gas In Physics.
From www.slideserve.com
PPT Ideal Gas Law PowerPoint Presentation, free download ID7060016 Ideal Gas In Physics Learn about the ideal gas law, a general equation of state that relates the pressure, volume, and temperature of a gas. An ideal gas is a gas that obeys the equation \\ (pv = nkt\\), where \\ (p\\) is pressure, \\ (v\\) is volume, \\ (n\\) is number of molecules, and \\ (t\\) is temperature. See examples of how to. Ideal Gas In Physics.
From www.sliderbase.com
The Ideal Gas Law Presentation Chemistry Ideal Gas In Physics An ideal gas is a gas that obeys the equation \\ (pv = nkt\\), where \\ (p\\) is pressure, \\ (v\\) is volume, \\ (n\\) is number of molecules, and \\ (t\\) is temperature. Explore the properties and processes of ideal gases, such. Learn how the ideal gas law relates the pressure, volume, temperature, and number of atoms or molecules. Ideal Gas In Physics.
From chem-net.blogspot.com
Gas Laws Ideal Gas Law Chemistry Net Ideal Gas In Physics Learn how the ideal gas law relates the pressure, volume, temperature, and number of atoms or molecules of a gas. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Find out how to use the universal gas constant r and the kinetic. Learn. Ideal Gas In Physics.
From mmerevise.co.uk
The Ideal Gas Equation MME Ideal Gas In Physics Learn how the ideal gas law (pv=nrt or pv=nkt) is derived from empirical and microscopic considerations. Find out how to use the universal gas constant r and the kinetic. Explore the properties and processes of ideal gases, such. Learn about the ideal gas law, a general equation of state that relates the pressure, volume, and temperature of a gas. Learn. Ideal Gas In Physics.
From www.britannica.com
Ideal gas law Definition, Formula, & Facts Britannica Ideal Gas In Physics See examples of how to use the ideal gas law to calculate changes. Explore the properties and processes of ideal gases, such. Learn how the ideal gas law (pv=nrt or pv=nkt) is derived from empirical and microscopic considerations. An ideal gas is a gas that obeys the equation \\ (pv = nkt\\), where \\ (p\\) is pressure, \\ (v\\) is. Ideal Gas In Physics.
From www.dreamstime.com
Boyle Law Infographic Diagram Absolute Pressure Ideal Gas Volume Amount Ideal Gas In Physics See examples of how to use the ideal gas law to calculate changes. Explore the properties and processes of ideal gases, such. Learn how the ideal gas law (pv=nrt or pv=nkt) is derived from empirical and microscopic considerations. Learn about the ideal gas law, a general equation of state that relates the pressure, volume, and temperature of a gas. Learn. Ideal Gas In Physics.
From www.slideserve.com
PPT Ideal Gas Law & Gas Stoichiometry PowerPoint Presentation ID Ideal Gas In Physics Explore the properties and processes of ideal gases, such. An ideal gas is a gas that obeys the equation \\ (pv = nkt\\), where \\ (p\\) is pressure, \\ (v\\) is volume, \\ (n\\) is number of molecules, and \\ (t\\) is temperature. Learn how the ideal gas law (pv=nrt or pv=nkt) is derived from empirical and microscopic considerations. Ideal. Ideal Gas In Physics.
From www.slideserve.com
PPT Chapter 10 Gases & the Atmosphere PowerPoint Presentation ID611285 Ideal Gas In Physics See examples of how to use the ideal gas law to calculate changes. Learn how the ideal gas law (pv=nrt or pv=nkt) is derived from empirical and microscopic considerations. Learn how the ideal gas law relates the pressure, volume, temperature, and number of atoms or molecules of a gas. Ideal gas, a gas that conforms, in physical behavior, to a. Ideal Gas In Physics.
From www.expii.com
Ideal Gas Law — Overview & Calculations Expii Ideal Gas In Physics Learn how the ideal gas law (pv=nrt or pv=nkt) is derived from empirical and microscopic considerations. Find out how to use the universal gas constant r and the kinetic. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Explore the properties and processes. Ideal Gas In Physics.
From www.youtube.com
1.2 The Ideal Gas (Thermal Physics) (Schroeder) YouTube Ideal Gas In Physics An ideal gas is a gas that obeys the equation \\ (pv = nkt\\), where \\ (p\\) is pressure, \\ (v\\) is volume, \\ (n\\) is number of molecules, and \\ (t\\) is temperature. Explore the properties and processes of ideal gases, such. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume,. Ideal Gas In Physics.
From www.britannica.com
Boyle’s law Definition, Equation, & Facts Britannica Ideal Gas In Physics Learn how the ideal gas law (pv=nrt or pv=nkt) is derived from empirical and microscopic considerations. Find out how to use the universal gas constant r and the kinetic. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. An ideal gas is a. Ideal Gas In Physics.
From www.slideserve.com
PPT Reaction Equilibrium in Ideal Gas Mixture PowerPoint Presentation Ideal Gas In Physics Learn how the ideal gas law relates the pressure, volume, temperature, and number of atoms or molecules of a gas. An ideal gas is a gas that obeys the equation \\ (pv = nkt\\), where \\ (p\\) is pressure, \\ (v\\) is volume, \\ (n\\) is number of molecules, and \\ (t\\) is temperature. Find out how to use the. Ideal Gas In Physics.
From www.alamy.com
Ideal gas law formula. Pressure, volume, amount of substance , ideal Ideal Gas In Physics Learn how the ideal gas law (pv=nrt or pv=nkt) is derived from empirical and microscopic considerations. Explore the properties and processes of ideal gases, such. Learn about the ideal gas law, a general equation of state that relates the pressure, volume, and temperature of a gas. An ideal gas is a gas that obeys the equation \\ (pv = nkt\\),. Ideal Gas In Physics.
From www.slideserve.com
PPT Thermal Physics (Thermodynamics) PowerPoint Presentation ID421070 Ideal Gas In Physics Learn about the ideal gas law, a general equation of state that relates the pressure, volume, and temperature of a gas. An ideal gas is a gas that obeys the equation \\ (pv = nkt\\), where \\ (p\\) is pressure, \\ (v\\) is volume, \\ (n\\) is number of molecules, and \\ (t\\) is temperature. Ideal gas, a gas that. Ideal Gas In Physics.
From www.science-revision.co.uk
The ideal gas equation Ideal Gas In Physics Learn about the ideal gas law, a general equation of state that relates the pressure, volume, and temperature of a gas. Learn how the ideal gas law (pv=nrt or pv=nkt) is derived from empirical and microscopic considerations. An ideal gas is a gas that obeys the equation \\ (pv = nkt\\), where \\ (p\\) is pressure, \\ (v\\) is volume,. Ideal Gas In Physics.
From byjus.com
Ideal Gas Law Equation Compressibility Of Natural Gas Chemistry Ideal Gas In Physics Find out how to use the universal gas constant r and the kinetic. An ideal gas is a gas that obeys the equation \\ (pv = nkt\\), where \\ (p\\) is pressure, \\ (v\\) is volume, \\ (n\\) is number of molecules, and \\ (t\\) is temperature. Explore the properties and processes of ideal gases, such. See examples of how. Ideal Gas In Physics.
From www.slideserve.com
PPT Gases PowerPoint Presentation, free download ID4387987 Ideal Gas In Physics See examples of how to use the ideal gas law to calculate changes. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Learn about the ideal gas law, a general equation of state that relates the pressure, volume, and temperature of a gas.. Ideal Gas In Physics.
From www.slideserve.com
PPT Ideal Gas Equation PowerPoint Presentation, free download ID Ideal Gas In Physics Explore the properties and processes of ideal gases, such. Learn how the ideal gas law relates the pressure, volume, temperature, and number of atoms or molecules of a gas. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. See examples of how to. Ideal Gas In Physics.
From www.youtube.com
Ideal Gas Law Physics Problems With Boltzmann's Constant YouTube Ideal Gas In Physics An ideal gas is a gas that obeys the equation \\ (pv = nkt\\), where \\ (p\\) is pressure, \\ (v\\) is volume, \\ (n\\) is number of molecules, and \\ (t\\) is temperature. Learn how the ideal gas law relates the pressure, volume, temperature, and number of atoms or molecules of a gas. Ideal gas, a gas that conforms,. Ideal Gas In Physics.
From www.grc.nasa.gov
Equation of State Ideal Gas In Physics See examples of how to use the ideal gas law to calculate changes. Learn how the ideal gas law (pv=nrt or pv=nkt) is derived from empirical and microscopic considerations. Learn how the ideal gas law relates the pressure, volume, temperature, and number of atoms or molecules of a gas. Explore the properties and processes of ideal gases, such. Find out. Ideal Gas In Physics.
From mathformula5.netlify.app
What Is The Mathematical Formula Of Boyles Law Complete Guide Ideal Gas In Physics See examples of how to use the ideal gas law to calculate changes. Learn how the ideal gas law relates the pressure, volume, temperature, and number of atoms or molecules of a gas. Learn about the ideal gas law, a general equation of state that relates the pressure, volume, and temperature of a gas. Learn how the ideal gas law. Ideal Gas In Physics.
From mmerevise.co.uk
Ideal Gases Worksheets, Questions and Revision MME Ideal Gas In Physics Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Learn how the ideal gas law relates the pressure, volume, temperature, and number of atoms or molecules of a gas. An ideal gas is a gas that obeys the equation \\ (pv = nkt\\),. Ideal Gas In Physics.
From www.dreamstime.com
Ideal Gas Law. Boyles Law Pressure Volume Relationship in Gases Stock Ideal Gas In Physics See examples of how to use the ideal gas law to calculate changes. Learn how the ideal gas law relates the pressure, volume, temperature, and number of atoms or molecules of a gas. Learn about the ideal gas law, a general equation of state that relates the pressure, volume, and temperature of a gas. Ideal gas, a gas that conforms,. Ideal Gas In Physics.
From www.slideserve.com
PPT The Combined and Ideal Gas Laws PowerPoint Presentation, free Ideal Gas In Physics See examples of how to use the ideal gas law to calculate changes. An ideal gas is a gas that obeys the equation \\ (pv = nkt\\), where \\ (p\\) is pressure, \\ (v\\) is volume, \\ (n\\) is number of molecules, and \\ (t\\) is temperature. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized. Ideal Gas In Physics.
From owlcation.com
The Theories and Behavior of Gas Owlcation Ideal Gas In Physics Learn how the ideal gas law (pv=nrt or pv=nkt) is derived from empirical and microscopic considerations. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. An ideal gas is a gas that obeys the equation \\ (pv = nkt\\), where \\ (p\\) is. Ideal Gas In Physics.
From www.youtube.com
15.1a Ideal Gas Law (Theory) A2 Cambridge A Level 9702 Physics Ideal Gas In Physics Explore the properties and processes of ideal gases, such. Learn how the ideal gas law (pv=nrt or pv=nkt) is derived from empirical and microscopic considerations. An ideal gas is a gas that obeys the equation \\ (pv = nkt\\), where \\ (p\\) is pressure, \\ (v\\) is volume, \\ (n\\) is number of molecules, and \\ (t\\) is temperature. Learn. Ideal Gas In Physics.
From www.slideserve.com
PPT Physics 101 Lecture 29 Ideal Gas Law & Theory PowerPoint Ideal Gas In Physics Learn how the ideal gas law relates the pressure, volume, temperature, and number of atoms or molecules of a gas. Learn how the ideal gas law (pv=nrt or pv=nkt) is derived from empirical and microscopic considerations. Learn about the ideal gas law, a general equation of state that relates the pressure, volume, and temperature of a gas. See examples of. Ideal Gas In Physics.
From app.jove.com
Ideal Gas Equation Concept Physics JoVe Ideal Gas In Physics Explore the properties and processes of ideal gases, such. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Find out how to use the universal gas constant r and the kinetic. An ideal gas is a gas that obeys the equation \\ (pv. Ideal Gas In Physics.
From www.slideserve.com
PPT Ideal Gas Equation PowerPoint Presentation, free download ID Ideal Gas In Physics Explore the properties and processes of ideal gases, such. Ideal gas, a gas that conforms, in physical behavior, to a particular idealized relation between pressure, volume, and temperature called the ideal gas law, which states. Learn how the ideal gas law relates the pressure, volume, temperature, and number of atoms or molecules of a gas. Learn how the ideal gas. Ideal Gas In Physics.
From www.slideserve.com
PPT C H A P T E R 14 The Ideal Gas Law and Theory PowerPoint Ideal Gas In Physics Explore the properties and processes of ideal gases, such. An ideal gas is a gas that obeys the equation \\ (pv = nkt\\), where \\ (p\\) is pressure, \\ (v\\) is volume, \\ (n\\) is number of molecules, and \\ (t\\) is temperature. Learn how the ideal gas law (pv=nrt or pv=nkt) is derived from empirical and microscopic considerations. See. Ideal Gas In Physics.
From www.alamy.com
Ideal gas law, illustration Stock Photo Alamy Ideal Gas In Physics See examples of how to use the ideal gas law to calculate changes. Learn how the ideal gas law relates the pressure, volume, temperature, and number of atoms or molecules of a gas. Learn about the ideal gas law, a general equation of state that relates the pressure, volume, and temperature of a gas. An ideal gas is a gas. Ideal Gas In Physics.