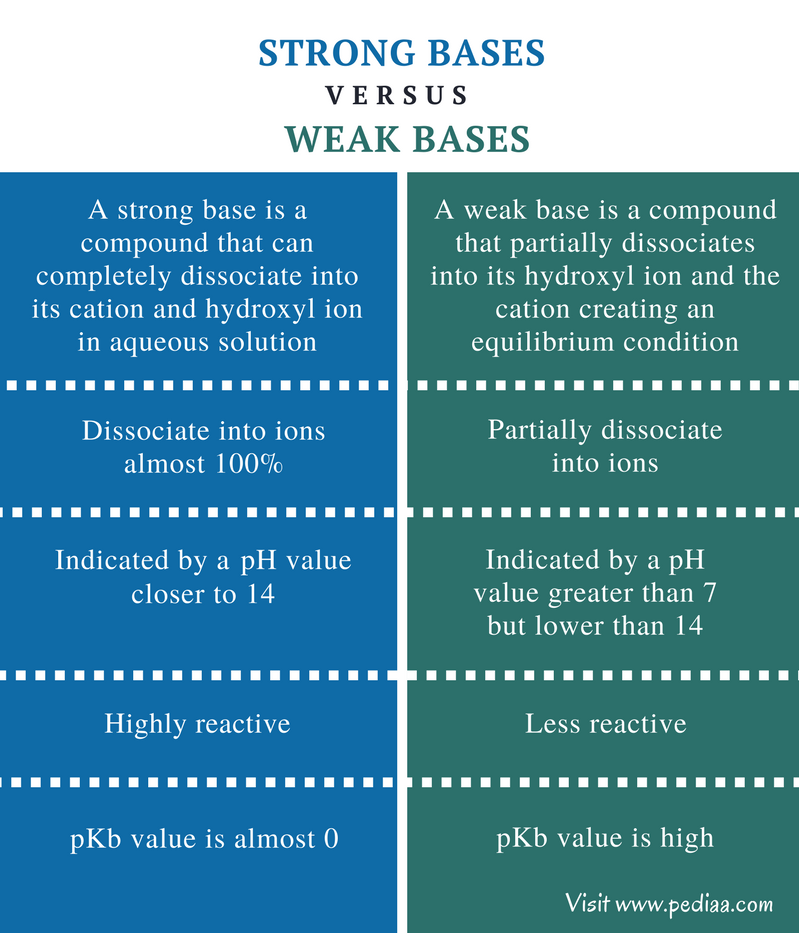
Strong Base Definition Chemistry . If it is less than 100%. You can think of the compound as being 100% split. A strong base is a base that is completely dissociated in an aqueous solution, such as sodium hydroxide or potassium hydroxide. Learn what strong bases are, how they dissociate in water, and what properties they have. A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. Find out the most common strong bases, such as hydroxides of alkali and alkaline earth. Learn the names, formulas, and properties of strong acids and bases, and how to calculate their ph and poh. When you dissolve bases in water, they increase the. A strong base is a base that is 100% ionized in solution. The issue is similar with bases: In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs of electrons.
from pediaa.com
If it is less than 100%. You can think of the compound as being 100% split. In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs of electrons. A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. A strong base is a base that is completely dissociated in an aqueous solution, such as sodium hydroxide or potassium hydroxide. A strong base is a base that is 100% ionized in solution. The issue is similar with bases: Learn what strong bases are, how they dissociate in water, and what properties they have. Learn the names, formulas, and properties of strong acids and bases, and how to calculate their ph and poh. When you dissolve bases in water, they increase the.
Difference Between Strong and Weak Bases Definition, Properties
Strong Base Definition Chemistry In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs of electrons. If it is less than 100%. Learn what strong bases are, how they dissociate in water, and what properties they have. A strong base is a base that is completely dissociated in an aqueous solution, such as sodium hydroxide or potassium hydroxide. Find out the most common strong bases, such as hydroxides of alkali and alkaline earth. A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. You can think of the compound as being 100% split. When you dissolve bases in water, they increase the. The issue is similar with bases: A strong base is a base that is 100% ionized in solution. Learn the names, formulas, and properties of strong acids and bases, and how to calculate their ph and poh. In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs of electrons.
From www.blendspace.com
Acids And Bases Introduction Lessons Blendspace Strong Base Definition Chemistry Learn the names, formulas, and properties of strong acids and bases, and how to calculate their ph and poh. Find out the most common strong bases, such as hydroxides of alkali and alkaline earth. When you dissolve bases in water, they increase the. You can think of the compound as being 100% split. In the world of chemistry, bases are. Strong Base Definition Chemistry.
From www.teachoo.com
List of Strong Bases 7+ Examples Chemistry Teachoo Strong Base Definition Chemistry A strong base is a base that is 100% ionized in solution. Learn the names, formulas, and properties of strong acids and bases, and how to calculate their ph and poh. A strong base is a base that is completely dissociated in an aqueous solution, such as sodium hydroxide or potassium hydroxide. If it is less than 100%. Learn what. Strong Base Definition Chemistry.
From www.youtube.com
pH of Strong Acids and Bases YouTube Strong Base Definition Chemistry A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. Learn the names, formulas, and properties of strong acids and bases, and how to calculate their ph and poh. When you dissolve bases in water, they increase the. A strong base is a base that is 100% ionized in solution. Find out the most common. Strong Base Definition Chemistry.
From www.teachoo.com
Examples of Weak Base (5 Examples with Images) Teachoo Chemistry Strong Base Definition Chemistry A strong base is a base that is completely dissociated in an aqueous solution, such as sodium hydroxide or potassium hydroxide. When you dissolve bases in water, they increase the. If it is less than 100%. You can think of the compound as being 100% split. Learn what strong bases are, how they dissociate in water, and what properties they. Strong Base Definition Chemistry.
From www.priyamstudycentre.com
Conjugate Acid Base pair Definition, Concept, Examples, List Strong Base Definition Chemistry If it is less than 100%. Learn the names, formulas, and properties of strong acids and bases, and how to calculate their ph and poh. A strong base is a base that is completely dissociated in an aqueous solution, such as sodium hydroxide or potassium hydroxide. When you dissolve bases in water, they increase the. Learn what strong bases are,. Strong Base Definition Chemistry.
From www.slideserve.com
PPT Acids & Bases PowerPoint Presentation ID6155898 Strong Base Definition Chemistry A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. You can think of the compound as being 100% split. A strong base is a base that is 100% ionized in solution. If it is less than 100%. Learn the names, formulas, and properties of strong acids and bases, and how to calculate their ph. Strong Base Definition Chemistry.
From www.slideserve.com
PPT Acids and Bases PowerPoint Presentation, free download ID2089759 Strong Base Definition Chemistry The issue is similar with bases: A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. Find out the most common strong bases, such as hydroxides of alkali and alkaline earth. When you dissolve bases in water, they increase the. Learn what strong bases are, how they dissociate in water, and what properties they have.. Strong Base Definition Chemistry.
From www.slideserve.com
PPT Acids and Bases pH and Titrations PowerPoint Presentation, free Strong Base Definition Chemistry When you dissolve bases in water, they increase the. Find out the most common strong bases, such as hydroxides of alkali and alkaline earth. In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs of electrons. A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. You. Strong Base Definition Chemistry.
From www.slideserve.com
PPT Strong Acids and Bases PowerPoint Presentation, free download Strong Base Definition Chemistry A strong base is a base that is 100% ionized in solution. When you dissolve bases in water, they increase the. A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. A strong base is a base that is completely dissociated in an aqueous solution, such as sodium hydroxide or potassium hydroxide. Find out the. Strong Base Definition Chemistry.
From www.teachoo.com
Difference between Strong and Weak Base with Examples [in Table] Strong Base Definition Chemistry The issue is similar with bases: A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. A strong base is a base that is completely dissociated in an aqueous solution, such as sodium hydroxide or potassium hydroxide. In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs. Strong Base Definition Chemistry.
From www.slideserve.com
PPT Acids, Bases and pH PowerPoint Presentation, free download ID Strong Base Definition Chemistry A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. You can think of the compound as being 100% split. A strong base is a base that is completely dissociated in an aqueous solution, such as sodium hydroxide or potassium hydroxide. Find out the most common strong bases, such as hydroxides of alkali and alkaline. Strong Base Definition Chemistry.
From pediaa.com
Difference Between Strong and Weak Bases Definition, Properties Strong Base Definition Chemistry A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. The issue is similar with bases: A strong base is a base that is completely dissociated in an aqueous solution, such as sodium hydroxide or potassium hydroxide. Find out the most common strong bases, such as hydroxides of alkali and alkaline earth. In the world. Strong Base Definition Chemistry.
From www.worksheetsplanet.com
What is a Base Definition of Base Strong Base Definition Chemistry Learn the names, formulas, and properties of strong acids and bases, and how to calculate their ph and poh. If it is less than 100%. A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. The issue is similar with bases: In the world of chemistry, bases are substances that can accept protons (h +. Strong Base Definition Chemistry.
From www.thoughtco.com
List of the Strong Bases (Arrhenius Bases) Strong Base Definition Chemistry The issue is similar with bases: A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs of electrons. Find out the most common strong bases, such as hydroxides of alkali and alkaline earth. A strong base is. Strong Base Definition Chemistry.
From studylib.net
Strong and weak acids and bases Strong Base Definition Chemistry A strong base is a base that is 100% ionized in solution. A strong base is a base that is completely dissociated in an aqueous solution, such as sodium hydroxide or potassium hydroxide. When you dissolve bases in water, they increase the. You can think of the compound as being 100% split. Learn what strong bases are, how they dissociate. Strong Base Definition Chemistry.
From www.slideserve.com
PPT Chapter 14 Acids and Bases PowerPoint Presentation, free download Strong Base Definition Chemistry When you dissolve bases in water, they increase the. You can think of the compound as being 100% split. Learn what strong bases are, how they dissociate in water, and what properties they have. Learn the names, formulas, and properties of strong acids and bases, and how to calculate their ph and poh. A strong base is a base that. Strong Base Definition Chemistry.
From museuvirtual.injc.ufrj.br
Travaux ménagers je porte des vêtements Caractériser strong acids and Strong Base Definition Chemistry Find out the most common strong bases, such as hydroxides of alkali and alkaline earth. A strong base is a base that is completely dissociated in an aqueous solution, such as sodium hydroxide or potassium hydroxide. Learn the names, formulas, and properties of strong acids and bases, and how to calculate their ph and poh. When you dissolve bases in. Strong Base Definition Chemistry.
From www.slideserve.com
PPT The Chemistry of Acids and Bases PowerPoint Presentation, free Strong Base Definition Chemistry The issue is similar with bases: Learn what strong bases are, how they dissociate in water, and what properties they have. You can think of the compound as being 100% split. A strong base is a base that is 100% ionized in solution. Find out the most common strong bases, such as hydroxides of alkali and alkaline earth. In the. Strong Base Definition Chemistry.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs Strong Base Definition Chemistry If it is less than 100%. Learn the names, formulas, and properties of strong acids and bases, and how to calculate their ph and poh. The issue is similar with bases: When you dissolve bases in water, they increase the. A strong base is a base that is 100% ionized in solution. Learn what strong bases are, how they dissociate. Strong Base Definition Chemistry.
From leah4sci.com
Definitions of Arrhenius, BronstedLowry, and Lewis Acids and Bases in Strong Base Definition Chemistry The issue is similar with bases: A strong base is a base that is 100% ionized in solution. Learn the names, formulas, and properties of strong acids and bases, and how to calculate their ph and poh. You can think of the compound as being 100% split. Learn what strong bases are, how they dissociate in water, and what properties. Strong Base Definition Chemistry.
From www.pinterest.jp
Strong and Weak Acids & Bases Chemistry classroom, Ap chemistry Strong Base Definition Chemistry Learn the names, formulas, and properties of strong acids and bases, and how to calculate their ph and poh. A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. When you dissolve bases in water, they increase the. A strong base is a base that is completely dissociated in an aqueous solution, such as sodium. Strong Base Definition Chemistry.
From www.slideserve.com
PPT Bases PowerPoint Presentation, free download ID4763941 Strong Base Definition Chemistry The issue is similar with bases: Learn what strong bases are, how they dissociate in water, and what properties they have. A strong base is a base that is completely dissociated in an aqueous solution, such as sodium hydroxide or potassium hydroxide. When you dissolve bases in water, they increase the. In the world of chemistry, bases are substances that. Strong Base Definition Chemistry.
From www.teachoo.com
List of Strong Acids (More than 8 examples, with images) Teachoo Strong Base Definition Chemistry When you dissolve bases in water, they increase the. Learn what strong bases are, how they dissociate in water, and what properties they have. In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs of electrons. Learn the names, formulas, and properties of strong acids and bases, and how to calculate their. Strong Base Definition Chemistry.
From shiken.ai
Weak Acids and Bases Strong Base Definition Chemistry If it is less than 100%. Find out the most common strong bases, such as hydroxides of alkali and alkaline earth. The issue is similar with bases: In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs of electrons. Learn the names, formulas, and properties of strong acids and bases, and how. Strong Base Definition Chemistry.
From www.thoughtco.com
List of the Strong Bases (Arrhenius Bases) Strong Base Definition Chemistry Learn what strong bases are, how they dissociate in water, and what properties they have. When you dissolve bases in water, they increase the. A strong base is a base that is completely dissociated in an aqueous solution, such as sodium hydroxide or potassium hydroxide. You can think of the compound as being 100% split. Learn the names, formulas, and. Strong Base Definition Chemistry.
From www.thoughtco.com
Strong Base Definition Chemistry Glossary Strong Base Definition Chemistry A strong base is a base that is completely dissociated in an aqueous solution, such as sodium hydroxide or potassium hydroxide. Learn the names, formulas, and properties of strong acids and bases, and how to calculate their ph and poh. If it is less than 100%. A strong base is a base that is 100% ionized in solution. Learn what. Strong Base Definition Chemistry.
From www.organicchemistrytutor.com
AcidBase Chemistry — Organic Chemistry Tutor Strong Base Definition Chemistry A strong base is a base that is 100% ionized in solution. Learn what strong bases are, how they dissociate in water, and what properties they have. Find out the most common strong bases, such as hydroxides of alkali and alkaline earth. In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs. Strong Base Definition Chemistry.
From www.teachoo.com
Bases and it's Properties (with Examples, Definition) Teachoo Strong Base Definition Chemistry Find out the most common strong bases, such as hydroxides of alkali and alkaline earth. Learn what strong bases are, how they dissociate in water, and what properties they have. A strong base is a base that is 100% ionized in solution. You can think of the compound as being 100% split. When you dissolve bases in water, they increase. Strong Base Definition Chemistry.
From sciencenotes.org
What Is a Base in Chemistry? Definition and Examples Strong Base Definition Chemistry You can think of the compound as being 100% split. Learn the names, formulas, and properties of strong acids and bases, and how to calculate their ph and poh. A strong base is a base that is completely dissociated in an aqueous solution, such as sodium hydroxide or potassium hydroxide. A strong base is a base that is 100% ionized. Strong Base Definition Chemistry.
From www.geeksforgeeks.org
What are Bases? Definition, Examples, Types, Properties and Uses Strong Base Definition Chemistry You can think of the compound as being 100% split. Find out the most common strong bases, such as hydroxides of alkali and alkaline earth. Learn what strong bases are, how they dissociate in water, and what properties they have. A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. Learn the names, formulas, and. Strong Base Definition Chemistry.
From www.pinterest.co.kr
Strong Acids and Bases MCAT Chemistry Cheat Sheet Study Guide StudyPK Strong Base Definition Chemistry If it is less than 100%. Learn the names, formulas, and properties of strong acids and bases, and how to calculate their ph and poh. The issue is similar with bases: Find out the most common strong bases, such as hydroxides of alkali and alkaline earth. You can think of the compound as being 100% split. A strong base is. Strong Base Definition Chemistry.
From www.slideserve.com
PPT Acids & Bases PowerPoint Presentation, free download ID2011736 Strong Base Definition Chemistry A strong base is a base that is 100% ionized in solution. The issue is similar with bases: You can think of the compound as being 100% split. Find out the most common strong bases, such as hydroxides of alkali and alkaline earth. A strong base is a base that is completely dissociated in an aqueous solution, such as sodium. Strong Base Definition Chemistry.
From www.chemicals.co.uk
A Level Chemistry Revision Physical Chemistry Acids And Bases Strong Base Definition Chemistry In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs of electrons. Find out the most common strong bases, such as hydroxides of alkali and alkaline earth. When you dissolve bases in water, they increase the. Learn what strong bases are, how they dissociate in water, and what properties they have. A. Strong Base Definition Chemistry.
From blog.praxilabs.com
Learn All About The Strong Acids and Bases PraxiLabs Strong Base Definition Chemistry Learn what strong bases are, how they dissociate in water, and what properties they have. The issue is similar with bases: When you dissolve bases in water, they increase the. Find out the most common strong bases, such as hydroxides of alkali and alkaline earth. A strong base is a base that is 100% ionized in solution. A strong base. Strong Base Definition Chemistry.
From gamesmartz.com
Strong Base Definition & Image GameSmartz Strong Base Definition Chemistry In the world of chemistry, bases are substances that can accept protons (h + ions) or donate pairs of electrons. The issue is similar with bases: When you dissolve bases in water, they increase the. If it is less than 100%. A strong base is something like sodium hydroxide or potassium hydroxide which is fully ionic. Learn the names, formulas,. Strong Base Definition Chemistry.