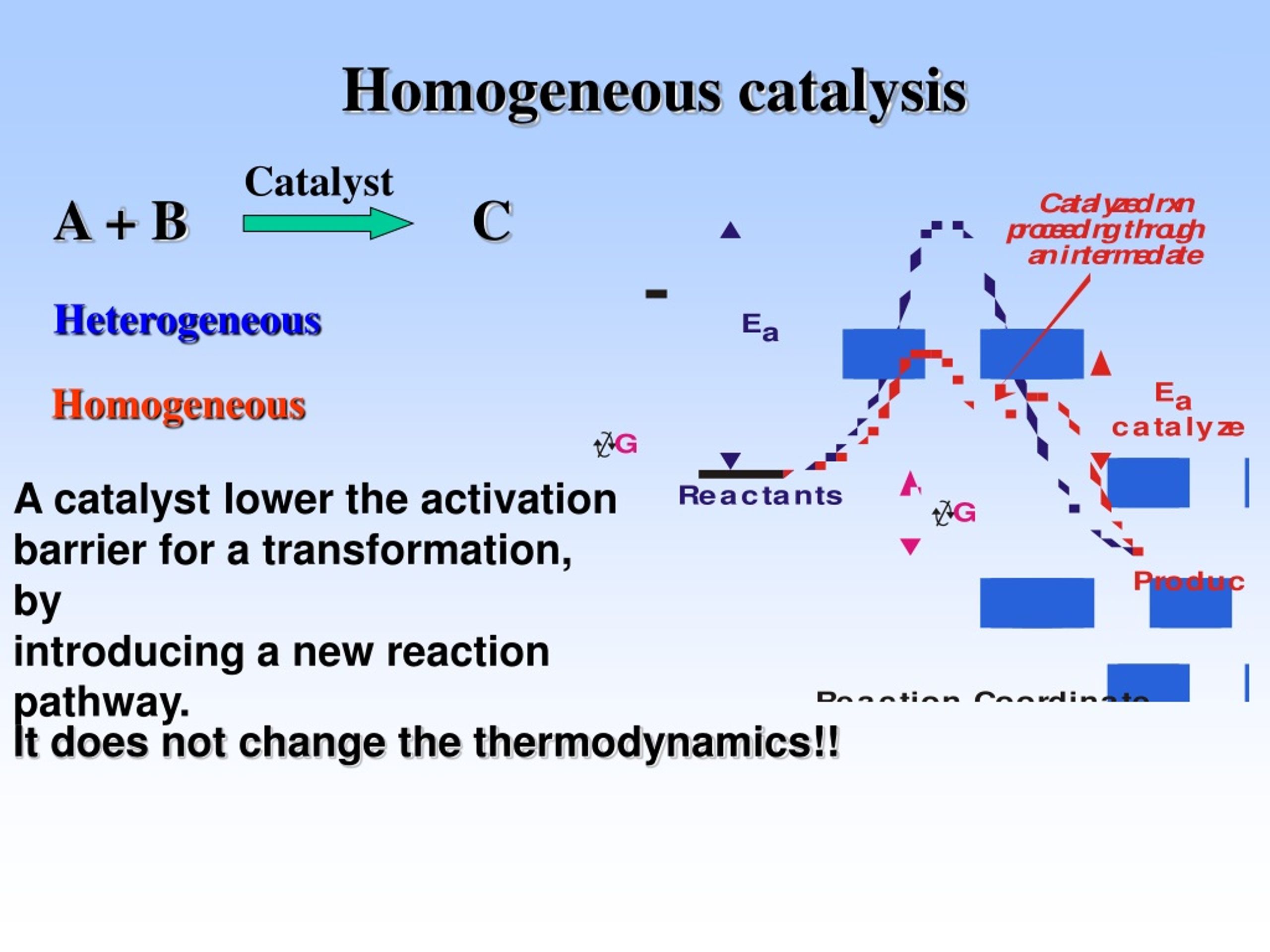
What Is Catalyst Class 10 With Example . Catalysis is the process of speeding up a reaction using a catalyst. The excess energy that the reactant molecules must acquire to change into the product is the activation energy. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. what are catalyst | types of catalyst | class 10 chemistry | chapter 01 ncertin this video, we'll explain what catalysts are in. in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. British chemistry elizabeth fulhame first. catalysis is the phenomena of altering the velocity of a chemical reaction by the presence of a catalyst. in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. Here are some important catalyst types and examples of catalyst in chemistry. 4.0 types of catalysts with examples. a catalyst is a substance that causes a chemical reaction to happen in a different way than it would happen without that. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. The temperature at which the catalyst activity is maximum is the optimum temp richer. In general, catalytic action is a chemical reaction between the catalyst and a reactant.
from exobghamq.blob.core.windows.net
The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. catalysis is the phenomena of altering the velocity of a chemical reaction by the presence of a catalyst. In general, catalytic action is a chemical reaction between the catalyst and a reactant. in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. what are catalyst | types of catalyst | class 10 chemistry | chapter 01 ncertin this video, we'll explain what catalysts are in. Here are some important catalyst types and examples of catalyst in chemistry. in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. 4.0 types of catalysts with examples. Catalysis is the process of speeding up a reaction using a catalyst.
Catalysts Chemical Equation at Joy Perry blog
What Is Catalyst Class 10 With Example Catalysis is the process of speeding up a reaction using a catalyst. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. British chemistry elizabeth fulhame first. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. catalysis is the phenomena of altering the velocity of a chemical reaction by the presence of a catalyst. in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. Catalysis is the process of speeding up a reaction using a catalyst. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In general, catalytic action is a chemical reaction between the catalyst and a reactant. what are catalyst | types of catalyst | class 10 chemistry | chapter 01 ncertin this video, we'll explain what catalysts are in. The excess energy that the reactant molecules must acquire to change into the product is the activation energy. 4.0 types of catalysts with examples. a catalyst is a substance that causes a chemical reaction to happen in a different way than it would happen without that. The temperature at which the catalyst activity is maximum is the optimum temp richer. Here are some important catalyst types and examples of catalyst in chemistry. in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it.
From www.youtube.com
class 10 lecture 1 (chemical reaction and catalyst) YouTube What Is Catalyst Class 10 With Example Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. a catalyst is a substance that causes a chemical reaction to happen in a different way than it would happen without that. In general, catalytic action is a chemical reaction between the catalyst and a reactant. catalyst, in chemistry, any substance that increases the rate of a. What Is Catalyst Class 10 With Example.
From www.youtube.com
How does a CATALYST work ? YouTube What Is Catalyst Class 10 With Example The excess energy that the reactant molecules must acquire to change into the product is the activation energy. catalysis is the phenomena of altering the velocity of a chemical reaction by the presence of a catalyst. Here are some important catalyst types and examples of catalyst in chemistry. 4.0 types of catalysts with examples. Catalysis is the process. What Is Catalyst Class 10 With Example.
From exowzdqkr.blob.core.windows.net
Catalyst Biology Image at Anna Schofield blog What Is Catalyst Class 10 With Example catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. 4.0 types of catalysts with examples. Catalysis is the process of speeding up a reaction using a catalyst. what are catalyst | types of catalyst | class 10 chemistry | chapter 01 ncertin this video, we'll explain what catalysts are in.. What Is Catalyst Class 10 With Example.
From exobghamq.blob.core.windows.net
Catalysts Chemical Equation at Joy Perry blog What Is Catalyst Class 10 With Example catalysis is the phenomena of altering the velocity of a chemical reaction by the presence of a catalyst. Catalysis is the process of speeding up a reaction using a catalyst. British chemistry elizabeth fulhame first. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. The temperature at which the catalyst activity is maximum is the optimum temp. What Is Catalyst Class 10 With Example.
From www.youtube.com
Chemical reactionsWHAT IS CATALYST? TYPES OF CATALYST RBSE CLASS What Is Catalyst Class 10 With Example The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. Catalysis is the process of speeding up a reaction using a catalyst. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. in chemistry and biology, a catalyst is a substance the increases the rate of a. What Is Catalyst Class 10 With Example.
From www.slideshare.net
Heterogeneous catalysisFundamentals What Is Catalyst Class 10 With Example The temperature at which the catalyst activity is maximum is the optimum temp richer. Catalysis is the process of speeding up a reaction using a catalyst. in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. British chemistry elizabeth fulhame first. catalyst, in chemistry, any substance. What Is Catalyst Class 10 With Example.
From sciencenotes.org
What Is a Catalyst? Understand Catalysis What Is Catalyst Class 10 With Example catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. catalysis is the phenomena of altering the velocity of a chemical reaction by the presence of a catalyst. a catalyst is a substance that causes a chemical reaction to happen. What Is Catalyst Class 10 With Example.
From www.youtube.com
Properties of Catalyst Chemistry Class 10 RBSE By Er. Lucky What Is Catalyst Class 10 With Example in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. British chemistry elizabeth fulhame first. what are catalyst | types of catalyst | class 10 chemistry | chapter 01 ncertin this video, we'll explain what catalysts are in. catalysis is the phenomena of altering the. What Is Catalyst Class 10 With Example.
From www.researchgate.net
Commonly Used Types of Catalysts and Their Range of Use Download What Is Catalyst Class 10 With Example 4.0 types of catalysts with examples. what are catalyst | types of catalyst | class 10 chemistry | chapter 01 ncertin this video, we'll explain what catalysts are in. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In general, catalytic action is a chemical reaction between the catalyst and. What Is Catalyst Class 10 With Example.
From dxotkfrql.blob.core.windows.net
What Is Catalyst Class 10 Cbse at Jesse Taber blog What Is Catalyst Class 10 With Example Here are some important catalyst types and examples of catalyst in chemistry. In general, catalytic action is a chemical reaction between the catalyst and a reactant. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. The excess energy that the reactant molecules must acquire to change into the product is the activation energy. The. What Is Catalyst Class 10 With Example.
From www.youtube.com
Catalyst Classes_CBSE_CLASS 10 ACIDS, BASES AND SALTS (CHEMISTRY What Is Catalyst Class 10 With Example 4.0 types of catalysts with examples. a catalyst is a substance that causes a chemical reaction to happen in a different way than it would happen without that. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. In general, catalytic action is a chemical reaction between the catalyst and a reactant. . What Is Catalyst Class 10 With Example.
From www.youtube.com
Practice Identifying Catalysts and Intermediates YouTube What Is Catalyst Class 10 With Example Here are some important catalyst types and examples of catalyst in chemistry. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. In general, catalytic action is a chemical reaction between the catalyst and a reactant.. What Is Catalyst Class 10 With Example.
From www.youtube.com
Class10 science chapter6 ,chemical reaction and catalyst YouTube What Is Catalyst Class 10 With Example in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. a catalyst is a substance that causes a chemical reaction to happen in a different way than it would happen without that. 4.0 types of catalysts with examples. The word “catalyst” comes from the greek word kataluein,. What Is Catalyst Class 10 With Example.
From www.worksheetsplanet.com
What is a Catalyst Definition of Catalyst What Is Catalyst Class 10 With Example Catalysis is the process of speeding up a reaction using a catalyst. in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. Here are some important catalyst types and examples of catalyst in chemistry. 4.0 types of catalysts with examples. The word “catalyst” comes from the greek word. What Is Catalyst Class 10 With Example.
From www.youtube.com
Class10 science chapter6 chemical reaction and catalyst YouTube What Is Catalyst Class 10 With Example British chemistry elizabeth fulhame first. Catalysis is the process of speeding up a reaction using a catalyst. catalysis is the phenomena of altering the velocity of a chemical reaction by the presence of a catalyst. The temperature at which the catalyst activity is maximum is the optimum temp richer. Enzymes are naturally occurring catalysts responsible for many essential biochemical. What Is Catalyst Class 10 With Example.
From www.slideserve.com
PPT CATALYSIS AND CATALYTIC REACTION MECHANISM PART 1 PowerPoint What Is Catalyst Class 10 With Example In general, catalytic action is a chemical reaction between the catalyst and a reactant. a catalyst is a substance that causes a chemical reaction to happen in a different way than it would happen without that. The excess energy that the reactant molecules must acquire to change into the product is the activation energy. what are catalyst |. What Is Catalyst Class 10 With Example.
From www.slideshare.net
Catalysis What Is Catalyst Class 10 With Example 4.0 types of catalysts with examples. In general, catalytic action is a chemical reaction between the catalyst and a reactant. The temperature at which the catalyst activity is maximum is the optimum temp richer. Catalysis is the process of speeding up a reaction using a catalyst. The word “catalyst” comes from the greek word kataluein, which means to loosen. What Is Catalyst Class 10 With Example.
From www.youtube.com
Class 10 Chapter 6 Chemical Reaction and Catalyst RBSE Science (Part4 What Is Catalyst Class 10 With Example British chemistry elizabeth fulhame first. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Here are some important catalyst types and examples of catalyst in chemistry. in chemistry and biology, a catalyst is a substance the increases the rate. What Is Catalyst Class 10 With Example.
From medicaltaste.weebly.com
Periodic table catalyst definition chemistry medicaltaste What Is Catalyst Class 10 With Example The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. a catalyst is a substance that causes a chemical reaction to happen in a different way than it would happen without that. in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by. What Is Catalyst Class 10 With Example.
From www.ck12.org
Catalysts Example 1 ( Video ) Chemistry CK12 Foundation What Is Catalyst Class 10 With Example The temperature at which the catalyst activity is maximum is the optimum temp richer. in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. 4.0 types of catalysts with examples. catalysis is the phenomena of altering the velocity of a chemical reaction by the presence. What Is Catalyst Class 10 With Example.
From www.teachoo.com
[Carbon Class 10] Hydrogenation of oils Definition, Reaction What Is Catalyst Class 10 With Example in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. Catalysis is the process of speeding up a reaction using a catalyst. The temperature at which the catalyst activity is maximum is the optimum temp richer. Here are some important catalyst types and examples of catalyst in chemistry. The. What Is Catalyst Class 10 With Example.
From www.sciencelearn.org.nz
Chemical reactions and catalysts — Science Learning Hub What Is Catalyst Class 10 With Example Here are some important catalyst types and examples of catalyst in chemistry. British chemistry elizabeth fulhame first. The excess energy that the reactant molecules must acquire to change into the product is the activation energy. The temperature at which the catalyst activity is maximum is the optimum temp richer. The word “catalyst” comes from the greek word kataluein, which means. What Is Catalyst Class 10 With Example.
From www.slideserve.com
PPT Industrial catalysis PowerPoint Presentation, free download ID What Is Catalyst Class 10 With Example in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. The temperature at which the catalyst activity is maximum is the optimum temp richer. In general, catalytic action is a chemical reaction between the. What Is Catalyst Class 10 With Example.
From slideplayer.com
Catalysts May SCH4U1. ppt download What Is Catalyst Class 10 With Example 4.0 types of catalysts with examples. in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. In general, catalytic action is a chemical reaction between the catalyst and a reactant. catalysis is the phenomena of altering the velocity of a chemical reaction by the presence of a. What Is Catalyst Class 10 With Example.
From www.youtube.com
Effect of Various Catalysts on Hydrogen Peroxide GCSE Chemistry YouTube What Is Catalyst Class 10 With Example what are catalyst | types of catalyst | class 10 chemistry | chapter 01 ncertin this video, we'll explain what catalysts are in. a catalyst is a substance that causes a chemical reaction to happen in a different way than it would happen without that. The excess energy that the reactant molecules must acquire to change into the. What Is Catalyst Class 10 With Example.
From dxomuorny.blob.core.windows.net
Catalyst In Chemistry Examples at Alton Stonge blog What Is Catalyst Class 10 With Example Here are some important catalyst types and examples of catalyst in chemistry. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. In general, catalytic action is a chemical reaction between the catalyst and a reactant. The word “catalyst” comes from the greek word kataluein, which means to loosen or untie. a. What Is Catalyst Class 10 With Example.
From www.youtube.com
Identifying catalysts in a reaction YouTube What Is Catalyst Class 10 With Example Here are some important catalyst types and examples of catalyst in chemistry. Catalysis is the process of speeding up a reaction using a catalyst. In general, catalytic action is a chemical reaction between the catalyst and a reactant. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. in chemistry and biology, a catalyst is a substance the. What Is Catalyst Class 10 With Example.
From brainly.in
define catalyst with example ?? Brainly.in What Is Catalyst Class 10 With Example what are catalyst | types of catalyst | class 10 chemistry | chapter 01 ncertin this video, we'll explain what catalysts are in. The excess energy that the reactant molecules must acquire to change into the product is the activation energy. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. The word “catalyst” comes from the greek. What Is Catalyst Class 10 With Example.
From www.youtube.com
Chemical Reactions and Catalyst Class10(EM/HM) Part1 YouTube What Is Catalyst Class 10 With Example The excess energy that the reactant molecules must acquire to change into the product is the activation energy. in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. Enzymes are naturally occurring catalysts responsible for many essential biochemical reactions. catalyst, in chemistry, any substance that increases the rate. What Is Catalyst Class 10 With Example.
From www.slideshare.net
Catalyst What Is Catalyst Class 10 With Example Here are some important catalyst types and examples of catalyst in chemistry. The temperature at which the catalyst activity is maximum is the optimum temp richer. The excess energy that the reactant molecules must acquire to change into the product is the activation energy. Catalysis is the process of speeding up a reaction using a catalyst. In general, catalytic action. What Is Catalyst Class 10 With Example.
From exonprxge.blob.core.windows.net
Catalyst Examples In Life at Anthony Snyder blog What Is Catalyst Class 10 With Example in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. The temperature at which the catalyst activity is maximum is the optimum temp richer. 4.0 types of catalysts with examples. in chemistry, catalysts are defined as those substances which alter the rate of reaction by. What Is Catalyst Class 10 With Example.
From www.slideserve.com
PPT ENZYME BIOLOGICAL CATALYST PowerPoint Presentation, free What Is Catalyst Class 10 With Example in chemistry and biology, a catalyst is a substance the increases the rate of a chemical reaction without being consumed by it. in chemistry, catalysts are defined as those substances which alter the rate of reaction by changing the path of reaction. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being. What Is Catalyst Class 10 With Example.
From www.youtube.com
What Are Catalysts? Reactions Chemistry FuseSchool YouTube What Is Catalyst Class 10 With Example The temperature at which the catalyst activity is maximum is the optimum temp richer. In general, catalytic action is a chemical reaction between the catalyst and a reactant. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis is the process of speeding up a reaction using a catalyst. The excess energy. What Is Catalyst Class 10 With Example.
From www.slideserve.com
PPT Transition metals catalysts PowerPoint Presentation, free What Is Catalyst Class 10 With Example The temperature at which the catalyst activity is maximum is the optimum temp richer. Catalysis is the process of speeding up a reaction using a catalyst. catalysis is the phenomena of altering the velocity of a chemical reaction by the presence of a catalyst. British chemistry elizabeth fulhame first. 4.0 types of catalysts with examples. in chemistry. What Is Catalyst Class 10 With Example.
From exobfetmk.blob.core.windows.net
Examples Of Catalysts Gcse Chemistry at Colin Aleman blog What Is Catalyst Class 10 With Example what are catalyst | types of catalyst | class 10 chemistry | chapter 01 ncertin this video, we'll explain what catalysts are in. catalyst, in chemistry, any substance that increases the rate of a reaction without itself being consumed. Catalysis is the process of speeding up a reaction using a catalyst. Here are some important catalyst types and. What Is Catalyst Class 10 With Example.