Kp And Partial Pressure . Learn how to calculate and use the equilibrium constant kp based on the partial pressures of gases in a reaction. Find examples, definitions and formulas for equilibrium. It covers an explanation of the terms mole. Find \(k\) by writing each equilibrium constant expression as the ratio of the concentrations of the products and reactants,. Learn how to calculate kc and kp for chemical reactions involving gases and liquids, and how they depend on temperature. This page explains equilibrium constants expressed in terms of partial pressures of gases, k p. The equilibrium constant, kp, describes the ratio of reactants compared to products at equilibrium for a gaseous equilibrium system. Learn how to calculate the equilibrium constant (kp) for a reversible reaction involving gases, using partial pressures as. Learn about partial pressure, the pressure exerted by a component of a mixture of gases, and how it relates to the total pressure and the mole fraction of the component. See examples, definitions, and relationships with kc and chemical equations.
from www.numerade.com
Learn how to calculate the equilibrium constant (kp) for a reversible reaction involving gases, using partial pressures as. Find examples, definitions and formulas for equilibrium. See examples, definitions, and relationships with kc and chemical equations. Learn how to calculate and use the equilibrium constant kp based on the partial pressures of gases in a reaction. This page explains equilibrium constants expressed in terms of partial pressures of gases, k p. The equilibrium constant, kp, describes the ratio of reactants compared to products at equilibrium for a gaseous equilibrium system. Learn about partial pressure, the pressure exerted by a component of a mixture of gases, and how it relates to the total pressure and the mole fraction of the component. Learn how to calculate kc and kp for chemical reactions involving gases and liquids, and how they depend on temperature. It covers an explanation of the terms mole. Find \(k\) by writing each equilibrium constant expression as the ratio of the concentrations of the products and reactants,.
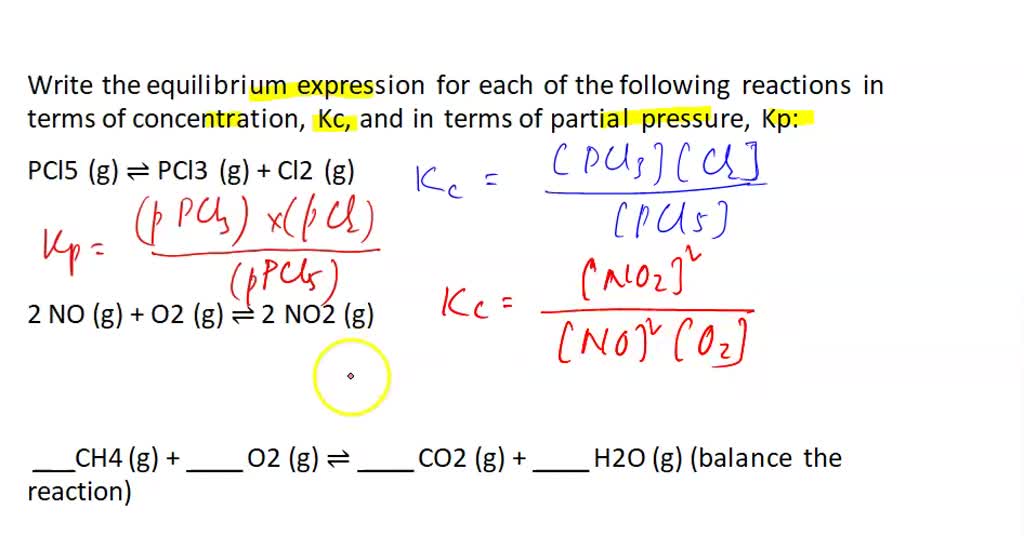
SOLVED Write the equilibrium expression for each of the following reactions in terms of
Kp And Partial Pressure See examples, definitions, and relationships with kc and chemical equations. It covers an explanation of the terms mole. Find \(k\) by writing each equilibrium constant expression as the ratio of the concentrations of the products and reactants,. The equilibrium constant, kp, describes the ratio of reactants compared to products at equilibrium for a gaseous equilibrium system. See examples, definitions, and relationships with kc and chemical equations. This page explains equilibrium constants expressed in terms of partial pressures of gases, k p. Find examples, definitions and formulas for equilibrium. Learn about partial pressure, the pressure exerted by a component of a mixture of gases, and how it relates to the total pressure and the mole fraction of the component. Learn how to calculate the equilibrium constant (kp) for a reversible reaction involving gases, using partial pressures as. Learn how to calculate and use the equilibrium constant kp based on the partial pressures of gases in a reaction. Learn how to calculate kc and kp for chemical reactions involving gases and liquids, and how they depend on temperature.
From www.youtube.com
Calculate Equilibrium Partial Pressure YouTube Kp And Partial Pressure Find \(k\) by writing each equilibrium constant expression as the ratio of the concentrations of the products and reactants,. Find examples, definitions and formulas for equilibrium. See examples, definitions, and relationships with kc and chemical equations. Learn how to calculate the equilibrium constant (kp) for a reversible reaction involving gases, using partial pressures as. Learn about partial pressure, the pressure. Kp And Partial Pressure.
From study.com
How to Calculate an Equilibrium Constant Kp Using Partial Pressures Chemistry Kp And Partial Pressure Find examples, definitions and formulas for equilibrium. Learn how to calculate the equilibrium constant (kp) for a reversible reaction involving gases, using partial pressures as. It covers an explanation of the terms mole. Learn how to calculate kc and kp for chemical reactions involving gases and liquids, and how they depend on temperature. Find \(k\) by writing each equilibrium constant. Kp And Partial Pressure.
From www.youtube.com
Kp Value Determination Effect of P, T on Kp Partial Pressure Determination using Kp A Kp And Partial Pressure Learn how to calculate kc and kp for chemical reactions involving gases and liquids, and how they depend on temperature. Learn how to calculate the equilibrium constant (kp) for a reversible reaction involving gases, using partial pressures as. See examples, definitions, and relationships with kc and chemical equations. The equilibrium constant, kp, describes the ratio of reactants compared to products. Kp And Partial Pressure.
From www.youtube.com
Equilibrium Part 6 ICE table with Kp problem YouTube Kp And Partial Pressure Find examples, definitions and formulas for equilibrium. The equilibrium constant, kp, describes the ratio of reactants compared to products at equilibrium for a gaseous equilibrium system. See examples, definitions, and relationships with kc and chemical equations. Learn how to calculate the equilibrium constant (kp) for a reversible reaction involving gases, using partial pressures as. This page explains equilibrium constants expressed. Kp And Partial Pressure.
From www.youtube.com
The equilibrium constant Kp YouTube Kp And Partial Pressure Find examples, definitions and formulas for equilibrium. Learn how to calculate the equilibrium constant (kp) for a reversible reaction involving gases, using partial pressures as. Learn about partial pressure, the pressure exerted by a component of a mixture of gases, and how it relates to the total pressure and the mole fraction of the component. The equilibrium constant, kp, describes. Kp And Partial Pressure.
From dokumen.tips
(PDF) 1.10 Partial Pressures and Kp Resources for Alevel · PDF file5/1/2016 · 1.10 Kp And Partial Pressure Find \(k\) by writing each equilibrium constant expression as the ratio of the concentrations of the products and reactants,. The equilibrium constant, kp, describes the ratio of reactants compared to products at equilibrium for a gaseous equilibrium system. Learn about partial pressure, the pressure exerted by a component of a mixture of gases, and how it relates to the total. Kp And Partial Pressure.
From www.youtube.com
Equilibrium, Kp and partial pressure YouTube Kp And Partial Pressure It covers an explanation of the terms mole. See examples, definitions, and relationships with kc and chemical equations. Learn how to calculate kc and kp for chemical reactions involving gases and liquids, and how they depend on temperature. Find \(k\) by writing each equilibrium constant expression as the ratio of the concentrations of the products and reactants,. The equilibrium constant,. Kp And Partial Pressure.
From www.youtube.com
Equilibrium Constant, Kp Partial Pressures Module 5 How Far? Chemistry A Level OCR A Kp And Partial Pressure See examples, definitions, and relationships with kc and chemical equations. It covers an explanation of the terms mole. The equilibrium constant, kp, describes the ratio of reactants compared to products at equilibrium for a gaseous equilibrium system. Learn how to calculate and use the equilibrium constant kp based on the partial pressures of gases in a reaction. Learn about partial. Kp And Partial Pressure.
From www.numerade.com
SOLVED Kp = 0.0198 at 721 K for the reaction 2HI (g) H2 (g) + I2 (g) In a particular experiment Kp And Partial Pressure Find examples, definitions and formulas for equilibrium. Find \(k\) by writing each equilibrium constant expression as the ratio of the concentrations of the products and reactants,. Learn about partial pressure, the pressure exerted by a component of a mixture of gases, and how it relates to the total pressure and the mole fraction of the component. Learn how to calculate. Kp And Partial Pressure.
From batubuayabradleys.blogspot.com
Calculate Equilibrium Partial Pressure Kp And Partial Pressure Learn how to calculate and use the equilibrium constant kp based on the partial pressures of gases in a reaction. Learn about partial pressure, the pressure exerted by a component of a mixture of gases, and how it relates to the total pressure and the mole fraction of the component. Find \(k\) by writing each equilibrium constant expression as the. Kp And Partial Pressure.
From www.numerade.com
SOLVED At 500 K the reaction PCl5(g) PCl3(g) + Cl2(g) has Kp = 0.497. In an equilibrium mixture Kp And Partial Pressure Learn about partial pressure, the pressure exerted by a component of a mixture of gases, and how it relates to the total pressure and the mole fraction of the component. It covers an explanation of the terms mole. Learn how to calculate the equilibrium constant (kp) for a reversible reaction involving gases, using partial pressures as. Find \(k\) by writing. Kp And Partial Pressure.
From www.numerade.com
SOLVED Write the equilibrium expression for each of the following reactions in terms of Kp And Partial Pressure See examples, definitions, and relationships with kc and chemical equations. Learn how to calculate kc and kp for chemical reactions involving gases and liquids, and how they depend on temperature. The equilibrium constant, kp, describes the ratio of reactants compared to products at equilibrium for a gaseous equilibrium system. Find \(k\) by writing each equilibrium constant expression as the ratio. Kp And Partial Pressure.
From www.tes.com
A2 Chem Kp, partial pressure and mole fractions. Teaching Resources Kp And Partial Pressure See examples, definitions, and relationships with kc and chemical equations. Learn how to calculate the equilibrium constant (kp) for a reversible reaction involving gases, using partial pressures as. Learn about partial pressure, the pressure exerted by a component of a mixture of gases, and how it relates to the total pressure and the mole fraction of the component. It covers. Kp And Partial Pressure.
From www.numerade.com
SOLVED Consider the following reaction A(g) ⇌ 2B(g). Find the equilibrium partial pressures Kp And Partial Pressure Learn about partial pressure, the pressure exerted by a component of a mixture of gases, and how it relates to the total pressure and the mole fraction of the component. Find examples, definitions and formulas for equilibrium. Learn how to calculate the equilibrium constant (kp) for a reversible reaction involving gases, using partial pressures as. See examples, definitions, and relationships. Kp And Partial Pressure.
From www.slideserve.com
PPT EQUILIBRIUM CONSTANTS Kp PowerPoint Presentation, free download ID6822448 Kp And Partial Pressure It covers an explanation of the terms mole. The equilibrium constant, kp, describes the ratio of reactants compared to products at equilibrium for a gaseous equilibrium system. See examples, definitions, and relationships with kc and chemical equations. Find examples, definitions and formulas for equilibrium. Learn how to calculate kc and kp for chemical reactions involving gases and liquids, and how. Kp And Partial Pressure.
From www.researchgate.net
Log of the partial pressure equilibrium constant Kp at specific... Download Scientific Diagram Kp And Partial Pressure See examples, definitions, and relationships with kc and chemical equations. Find \(k\) by writing each equilibrium constant expression as the ratio of the concentrations of the products and reactants,. Find examples, definitions and formulas for equilibrium. Learn how to calculate the equilibrium constant (kp) for a reversible reaction involving gases, using partial pressures as. This page explains equilibrium constants expressed. Kp And Partial Pressure.
From 9to5science.com
[Solved] Calculating equilibrium partial pressures of a 9to5Science Kp And Partial Pressure See examples, definitions, and relationships with kc and chemical equations. This page explains equilibrium constants expressed in terms of partial pressures of gases, k p. Find \(k\) by writing each equilibrium constant expression as the ratio of the concentrations of the products and reactants,. Learn how to calculate and use the equilibrium constant kp based on the partial pressures of. Kp And Partial Pressure.
From general.chemistrysteps.com
Kp Equilibrium Constant and Partial Pressure Chemistry Steps Kp And Partial Pressure It covers an explanation of the terms mole. This page explains equilibrium constants expressed in terms of partial pressures of gases, k p. Learn about partial pressure, the pressure exerted by a component of a mixture of gases, and how it relates to the total pressure and the mole fraction of the component. Learn how to calculate the equilibrium constant. Kp And Partial Pressure.
From www.slideserve.com
PPT Chemical Equilibrium PowerPoint Presentation, free download ID3098443 Kp And Partial Pressure Learn how to calculate kc and kp for chemical reactions involving gases and liquids, and how they depend on temperature. See examples, definitions, and relationships with kc and chemical equations. The equilibrium constant, kp, describes the ratio of reactants compared to products at equilibrium for a gaseous equilibrium system. This page explains equilibrium constants expressed in terms of partial pressures. Kp And Partial Pressure.
From chemistryguru.com.sg
Determine Kp given Reactant Partial Pressure Kp And Partial Pressure Learn how to calculate and use the equilibrium constant kp based on the partial pressures of gases in a reaction. See examples, definitions, and relationships with kc and chemical equations. Learn how to calculate the equilibrium constant (kp) for a reversible reaction involving gases, using partial pressures as. Learn how to calculate kc and kp for chemical reactions involving gases. Kp And Partial Pressure.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID6594850 Kp And Partial Pressure See examples, definitions, and relationships with kc and chemical equations. Learn how to calculate the equilibrium constant (kp) for a reversible reaction involving gases, using partial pressures as. This page explains equilibrium constants expressed in terms of partial pressures of gases, k p. Learn how to calculate and use the equilibrium constant kp based on the partial pressures of gases. Kp And Partial Pressure.
From learnah.org
Partial pressure and Kp Kp And Partial Pressure Find \(k\) by writing each equilibrium constant expression as the ratio of the concentrations of the products and reactants,. Learn how to calculate and use the equilibrium constant kp based on the partial pressures of gases in a reaction. Learn how to calculate kc and kp for chemical reactions involving gases and liquids, and how they depend on temperature. See. Kp And Partial Pressure.
From www.slideserve.com
PPT Chapter 14 Chemical Equilibrium PowerPoint Presentation, free download ID3053611 Kp And Partial Pressure Learn about partial pressure, the pressure exerted by a component of a mixture of gases, and how it relates to the total pressure and the mole fraction of the component. This page explains equilibrium constants expressed in terms of partial pressures of gases, k p. Learn how to calculate kc and kp for chemical reactions involving gases and liquids, and. Kp And Partial Pressure.
From brainly.in
How to calculate partial pressures if kp given? Brainly.in Kp And Partial Pressure The equilibrium constant, kp, describes the ratio of reactants compared to products at equilibrium for a gaseous equilibrium system. Find examples, definitions and formulas for equilibrium. Learn how to calculate kc and kp for chemical reactions involving gases and liquids, and how they depend on temperature. See examples, definitions, and relationships with kc and chemical equations. Learn how to calculate. Kp And Partial Pressure.
From www.numerade.com
SOLVED Consider the following equilibrium reaction A(g) + B (s) = C (g) If Kp = 6 * 103 Kp And Partial Pressure Learn how to calculate and use the equilibrium constant kp based on the partial pressures of gases in a reaction. The equilibrium constant, kp, describes the ratio of reactants compared to products at equilibrium for a gaseous equilibrium system. Find examples, definitions and formulas for equilibrium. This page explains equilibrium constants expressed in terms of partial pressures of gases, k. Kp And Partial Pressure.
From www.numerade.com
Consider the following reaction A(g)⇌2B(g) Find the equilibrium partial pressures of A and B Kp And Partial Pressure Learn about partial pressure, the pressure exerted by a component of a mixture of gases, and how it relates to the total pressure and the mole fraction of the component. The equilibrium constant, kp, describes the ratio of reactants compared to products at equilibrium for a gaseous equilibrium system. Find \(k\) by writing each equilibrium constant expression as the ratio. Kp And Partial Pressure.
From www.slideserve.com
PPT Chemistry 1011 PowerPoint Presentation, free download ID4449946 Kp And Partial Pressure Learn how to calculate and use the equilibrium constant kp based on the partial pressures of gases in a reaction. This page explains equilibrium constants expressed in terms of partial pressures of gases, k p. Find examples, definitions and formulas for equilibrium. The equilibrium constant, kp, describes the ratio of reactants compared to products at equilibrium for a gaseous equilibrium. Kp And Partial Pressure.
From chemistryguru.com.sg
Determine Kp given Reactant Partial Pressure Kp And Partial Pressure The equilibrium constant, kp, describes the ratio of reactants compared to products at equilibrium for a gaseous equilibrium system. Learn how to calculate and use the equilibrium constant kp based on the partial pressures of gases in a reaction. It covers an explanation of the terms mole. See examples, definitions, and relationships with kc and chemical equations. This page explains. Kp And Partial Pressure.
From www.numerade.com
SOLVED The equilibrium constant in terms of pressures, Kp for the reaction NHz(g) + HI(g) NHAI Kp And Partial Pressure Learn how to calculate and use the equilibrium constant kp based on the partial pressures of gases in a reaction. Find \(k\) by writing each equilibrium constant expression as the ratio of the concentrations of the products and reactants,. See examples, definitions, and relationships with kc and chemical equations. The equilibrium constant, kp, describes the ratio of reactants compared to. Kp And Partial Pressure.
From www.slideserve.com
PPT Equilibrium PowerPoint Presentation, free download ID654508 Kp And Partial Pressure The equilibrium constant, kp, describes the ratio of reactants compared to products at equilibrium for a gaseous equilibrium system. It covers an explanation of the terms mole. Learn how to calculate the equilibrium constant (kp) for a reversible reaction involving gases, using partial pressures as. Find examples, definitions and formulas for equilibrium. This page explains equilibrium constants expressed in terms. Kp And Partial Pressure.
From chemistryguru.com.sg
Determine Kp given Reactant Partial Pressure Kp And Partial Pressure Learn about partial pressure, the pressure exerted by a component of a mixture of gases, and how it relates to the total pressure and the mole fraction of the component. Learn how to calculate kc and kp for chemical reactions involving gases and liquids, and how they depend on temperature. Find examples, definitions and formulas for equilibrium. It covers an. Kp And Partial Pressure.
From www.youtube.com
Example Kp calculations YouTube Kp And Partial Pressure Find \(k\) by writing each equilibrium constant expression as the ratio of the concentrations of the products and reactants,. This page explains equilibrium constants expressed in terms of partial pressures of gases, k p. Find examples, definitions and formulas for equilibrium. It covers an explanation of the terms mole. Learn how to calculate and use the equilibrium constant kp based. Kp And Partial Pressure.
From www.youtube.com
10 9701_w04_qp_1 Partial Pressures, Kp mega Lecture YouTube Kp And Partial Pressure This page explains equilibrium constants expressed in terms of partial pressures of gases, k p. Learn how to calculate and use the equilibrium constant kp based on the partial pressures of gases in a reaction. Find \(k\) by writing each equilibrium constant expression as the ratio of the concentrations of the products and reactants,. Find examples, definitions and formulas for. Kp And Partial Pressure.
From www.nagwa.com
Question Video Calculating the Equilibrium Constant for Partial Pressures Given the Partial Kp And Partial Pressure It covers an explanation of the terms mole. Learn how to calculate and use the equilibrium constant kp based on the partial pressures of gases in a reaction. Learn how to calculate kc and kp for chemical reactions involving gases and liquids, and how they depend on temperature. Learn how to calculate the equilibrium constant (kp) for a reversible reaction. Kp And Partial Pressure.
From www.numerade.com
SOLVED At 500 K, the reaction PCl5(g) PCl3(g) + Cl2(g) has Kp = 0.497. In an equilibrium Kp And Partial Pressure The equilibrium constant, kp, describes the ratio of reactants compared to products at equilibrium for a gaseous equilibrium system. Learn how to calculate kc and kp for chemical reactions involving gases and liquids, and how they depend on temperature. This page explains equilibrium constants expressed in terms of partial pressures of gases, k p. Find \(k\) by writing each equilibrium. Kp And Partial Pressure.