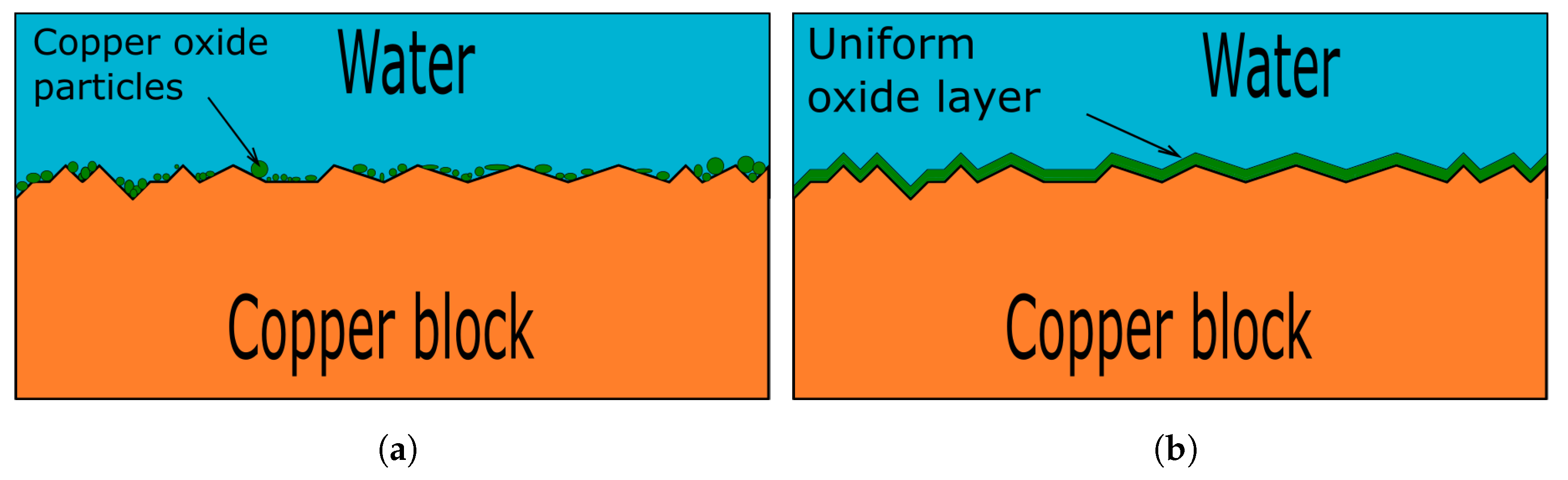
Copper Oxide + H2 . At higher temperatures the process is much faster. Natural gas (mainly methane) can also be used as a. Here oxidation state of cu in cuo is +2 and that of h in h2 is 0. Cuo + h2 → cu + h2o. Copper (ii) oxide and hydrogen gas. copper oxidizes slowly in air, corroding to produce a brown or green patina. Copper oxide hydrogen copper water. copper(ii) oxide can be reduced by hydrogen and its formula determined. cuo + h2 = cu2 + h2o is a single displacement (substitution) reaction where two moles of copper(ii) oxide [cuo] and. how to balance cuo + h2 = cu + h2o : determine the formula of copper(ii) oxide by reducing it using hydrogen or methane, in one of three methods available to you in this practical. the study of the reduction of copper oxide (cuo) by hydrogen (h 2) is helpful in elucidating the reduction mechanism of oxygen carriers.
from www.mdpi.com
Cuo + h2 → cu + h2o. copper oxidizes slowly in air, corroding to produce a brown or green patina. cuo + h2 = cu2 + h2o is a single displacement (substitution) reaction where two moles of copper(ii) oxide [cuo] and. Natural gas (mainly methane) can also be used as a. how to balance cuo + h2 = cu + h2o : At higher temperatures the process is much faster. Copper (ii) oxide and hydrogen gas. copper(ii) oxide can be reduced by hydrogen and its formula determined. Copper oxide hydrogen copper water. the study of the reduction of copper oxide (cuo) by hydrogen (h 2) is helpful in elucidating the reduction mechanism of oxygen carriers.
Applied Mechanics Free FullText Heat Transfer Deterioration by the
Copper Oxide + H2 Here oxidation state of cu in cuo is +2 and that of h in h2 is 0. At higher temperatures the process is much faster. Natural gas (mainly methane) can also be used as a. copper oxidizes slowly in air, corroding to produce a brown or green patina. copper(ii) oxide can be reduced by hydrogen and its formula determined. determine the formula of copper(ii) oxide by reducing it using hydrogen or methane, in one of three methods available to you in this practical. Copper oxide hydrogen copper water. Cuo + h2 → cu + h2o. Copper (ii) oxide and hydrogen gas. the study of the reduction of copper oxide (cuo) by hydrogen (h 2) is helpful in elucidating the reduction mechanism of oxygen carriers. cuo + h2 = cu2 + h2o is a single displacement (substitution) reaction where two moles of copper(ii) oxide [cuo] and. Here oxidation state of cu in cuo is +2 and that of h in h2 is 0. how to balance cuo + h2 = cu + h2o :
From exoxuauot.blob.core.windows.net
Copper Hydroxide Making at Josephine Reed blog Copper Oxide + H2 how to balance cuo + h2 = cu + h2o : the study of the reduction of copper oxide (cuo) by hydrogen (h 2) is helpful in elucidating the reduction mechanism of oxygen carriers. copper oxidizes slowly in air, corroding to produce a brown or green patina. Cuo + h2 → cu + h2o. Copper (ii) oxide. Copper Oxide + H2.
From www.sciencephoto.com
Copper (II) oxide reacts with hydrochloric acid Stock Image C036 Copper Oxide + H2 Here oxidation state of cu in cuo is +2 and that of h in h2 is 0. cuo + h2 = cu2 + h2o is a single displacement (substitution) reaction where two moles of copper(ii) oxide [cuo] and. how to balance cuo + h2 = cu + h2o : Natural gas (mainly methane) can also be used as. Copper Oxide + H2.
From www.youtube.com
CuO + H2 và Fe2O3 + H2. Hydrogen tác dụng với copper(II) oxide và iron Copper Oxide + H2 the study of the reduction of copper oxide (cuo) by hydrogen (h 2) is helpful in elucidating the reduction mechanism of oxygen carriers. how to balance cuo + h2 = cu + h2o : Here oxidation state of cu in cuo is +2 and that of h in h2 is 0. Copper oxide hydrogen copper water. copper(ii). Copper Oxide + H2.
From brainly.in
What happens when hydrogen gas is passed over black copper oxide Copper Oxide + H2 Copper (ii) oxide and hydrogen gas. Natural gas (mainly methane) can also be used as a. how to balance cuo + h2 = cu + h2o : cuo + h2 = cu2 + h2o is a single displacement (substitution) reaction where two moles of copper(ii) oxide [cuo] and. copper oxidizes slowly in air, corroding to produce a. Copper Oxide + H2.
From www.sciencephoto.com
Copper (II) oxide Stock Image C014/6955 Science Photo Library Copper Oxide + H2 how to balance cuo + h2 = cu + h2o : copper(ii) oxide can be reduced by hydrogen and its formula determined. Here oxidation state of cu in cuo is +2 and that of h in h2 is 0. Natural gas (mainly methane) can also be used as a. the study of the reduction of copper oxide. Copper Oxide + H2.
From www.mdpi.com
Applied Mechanics Free FullText Heat Transfer Deterioration by the Copper Oxide + H2 Cuo + h2 → cu + h2o. Natural gas (mainly methane) can also be used as a. copper oxidizes slowly in air, corroding to produce a brown or green patina. cuo + h2 = cu2 + h2o is a single displacement (substitution) reaction where two moles of copper(ii) oxide [cuo] and. copper(ii) oxide can be reduced by. Copper Oxide + H2.
From quizlet.com
Copper(II) oxide catalyses the of hydrogen per Quizlet Copper Oxide + H2 Copper (ii) oxide and hydrogen gas. At higher temperatures the process is much faster. copper oxidizes slowly in air, corroding to produce a brown or green patina. Cuo + h2 → cu + h2o. how to balance cuo + h2 = cu + h2o : Natural gas (mainly methane) can also be used as a. copper(ii) oxide. Copper Oxide + H2.
From pubs.acs.org
Understanding the Growth of Copper Oxide Nanowires and Layers by Copper Oxide + H2 the study of the reduction of copper oxide (cuo) by hydrogen (h 2) is helpful in elucidating the reduction mechanism of oxygen carriers. At higher temperatures the process is much faster. copper oxidizes slowly in air, corroding to produce a brown or green patina. Cuo + h2 → cu + h2o. Here oxidation state of cu in cuo. Copper Oxide + H2.
From www.numerade.com
SOLVED When copper(II) oxide is heated in hydrogen gas, the following Copper Oxide + H2 Natural gas (mainly methane) can also be used as a. determine the formula of copper(ii) oxide by reducing it using hydrogen or methane, in one of three methods available to you in this practical. Copper oxide hydrogen copper water. copper(ii) oxide can be reduced by hydrogen and its formula determined. cuo + h2 = cu2 + h2o. Copper Oxide + H2.
From www.slideserve.com
PPT Types Of Copper Oxide And Its Uses PowerPoint Presentation, free Copper Oxide + H2 Cuo + h2 → cu + h2o. Natural gas (mainly methane) can also be used as a. Copper (ii) oxide and hydrogen gas. determine the formula of copper(ii) oxide by reducing it using hydrogen or methane, in one of three methods available to you in this practical. the study of the reduction of copper oxide (cuo) by hydrogen. Copper Oxide + H2.
From www.youtube.com
How to Balance CuO + H2 = Cu + H2O Copper (II) Oxide and Hydrogen Gas Copper Oxide + H2 determine the formula of copper(ii) oxide by reducing it using hydrogen or methane, in one of three methods available to you in this practical. cuo + h2 = cu2 + h2o is a single displacement (substitution) reaction where two moles of copper(ii) oxide [cuo] and. At higher temperatures the process is much faster. copper(ii) oxide can be. Copper Oxide + H2.
From edu-rsc-org-s.webvpn.bjmu.doc110.com
Finding the formula of copper(II) oxide Experiment RSC Education Copper Oxide + H2 how to balance cuo + h2 = cu + h2o : determine the formula of copper(ii) oxide by reducing it using hydrogen or methane, in one of three methods available to you in this practical. the study of the reduction of copper oxide (cuo) by hydrogen (h 2) is helpful in elucidating the reduction mechanism of oxygen. Copper Oxide + H2.
From www.numerade.com
SOLVED Given the following reaction CuO (s) + H2 (g) Cu (s) + H2O (g Copper Oxide + H2 copper(ii) oxide can be reduced by hydrogen and its formula determined. At higher temperatures the process is much faster. Copper (ii) oxide and hydrogen gas. how to balance cuo + h2 = cu + h2o : copper oxidizes slowly in air, corroding to produce a brown or green patina. determine the formula of copper(ii) oxide by. Copper Oxide + H2.
From www.alamy.com
molecule, 3D illustration, copper oxide Stock Photo Alamy Copper Oxide + H2 Natural gas (mainly methane) can also be used as a. At higher temperatures the process is much faster. copper oxidizes slowly in air, corroding to produce a brown or green patina. how to balance cuo + h2 = cu + h2o : determine the formula of copper(ii) oxide by reducing it using hydrogen or methane, in one. Copper Oxide + H2.
From www.youtube.com
Copper(ii) Oxide Reduction using Hydrogen YouTube Copper Oxide + H2 determine the formula of copper(ii) oxide by reducing it using hydrogen or methane, in one of three methods available to you in this practical. copper(ii) oxide can be reduced by hydrogen and its formula determined. Cuo + h2 → cu + h2o. At higher temperatures the process is much faster. Copper oxide hydrogen copper water. Here oxidation state. Copper Oxide + H2.
From www.numerade.com
SOLVEDA mixture of oxygen and hydrogen is analyzed by passing it over Copper Oxide + H2 how to balance cuo + h2 = cu + h2o : Copper oxide hydrogen copper water. Copper (ii) oxide and hydrogen gas. Cuo + h2 → cu + h2o. copper(ii) oxide can be reduced by hydrogen and its formula determined. Natural gas (mainly methane) can also be used as a. cuo + h2 = cu2 + h2o. Copper Oxide + H2.
From edu.rsc.org
Reduction of copper(II) oxide by hydrogen Experiment RSC Education Copper Oxide + H2 copper oxidizes slowly in air, corroding to produce a brown or green patina. Copper oxide hydrogen copper water. copper(ii) oxide can be reduced by hydrogen and its formula determined. At higher temperatures the process is much faster. cuo + h2 = cu2 + h2o is a single displacement (substitution) reaction where two moles of copper(ii) oxide [cuo]. Copper Oxide + H2.
From testbook.com
Copper Hydroxide Learn Definition, Structure, Formula, Uses here Copper Oxide + H2 how to balance cuo + h2 = cu + h2o : Copper (ii) oxide and hydrogen gas. At higher temperatures the process is much faster. Here oxidation state of cu in cuo is +2 and that of h in h2 is 0. determine the formula of copper(ii) oxide by reducing it using hydrogen or methane, in one of. Copper Oxide + H2.
From www.cupricoxide.com
Wholesale Copper Oxide Paint Grade Manufacturer and Supplier, Factory Copper Oxide + H2 Cuo + h2 → cu + h2o. Here oxidation state of cu in cuo is +2 and that of h in h2 is 0. cuo + h2 = cu2 + h2o is a single displacement (substitution) reaction where two moles of copper(ii) oxide [cuo] and. Natural gas (mainly methane) can also be used as a. the study of. Copper Oxide + H2.
From brainly.in
to prepare copper oxide and to show the redox reaction of copper oxide Copper Oxide + H2 Copper (ii) oxide and hydrogen gas. Natural gas (mainly methane) can also be used as a. the study of the reduction of copper oxide (cuo) by hydrogen (h 2) is helpful in elucidating the reduction mechanism of oxygen carriers. how to balance cuo + h2 = cu + h2o : copper oxidizes slowly in air, corroding to. Copper Oxide + H2.
From www.sciencephoto.com
Copper Oxides Stock Image C028/0886 Science Photo Library Copper Oxide + H2 Here oxidation state of cu in cuo is +2 and that of h in h2 is 0. how to balance cuo + h2 = cu + h2o : At higher temperatures the process is much faster. copper(ii) oxide can be reduced by hydrogen and its formula determined. the study of the reduction of copper oxide (cuo) by. Copper Oxide + H2.
From www.slideserve.com
PPT Compounds & Elements PowerPoint Presentation, free download ID Copper Oxide + H2 cuo + h2 = cu2 + h2o is a single displacement (substitution) reaction where two moles of copper(ii) oxide [cuo] and. Copper (ii) oxide and hydrogen gas. copper oxidizes slowly in air, corroding to produce a brown or green patina. Here oxidation state of cu in cuo is +2 and that of h in h2 is 0. Cuo. Copper Oxide + H2.
From www.youtube.com
How to Write the Net Ionic Equation for CuO + HCl = CuCl2 + H2O YouTube Copper Oxide + H2 Cuo + h2 → cu + h2o. copper oxidizes slowly in air, corroding to produce a brown or green patina. Copper oxide hydrogen copper water. copper(ii) oxide can be reduced by hydrogen and its formula determined. how to balance cuo + h2 = cu + h2o : At higher temperatures the process is much faster. the. Copper Oxide + H2.
From slideplayer.com
(Things you should already know and remember) ppt download Copper Oxide + H2 Here oxidation state of cu in cuo is +2 and that of h in h2 is 0. how to balance cuo + h2 = cu + h2o : At higher temperatures the process is much faster. Copper oxide hydrogen copper water. Natural gas (mainly methane) can also be used as a. determine the formula of copper(ii) oxide by. Copper Oxide + H2.
From www.youtube.com
How to write the equation for CuO + H2O Copper (II) oxide + Water Copper Oxide + H2 Copper oxide hydrogen copper water. Natural gas (mainly methane) can also be used as a. Here oxidation state of cu in cuo is +2 and that of h in h2 is 0. At higher temperatures the process is much faster. Copper (ii) oxide and hydrogen gas. copper(ii) oxide can be reduced by hydrogen and its formula determined. Cuo +. Copper Oxide + H2.
From www.mdpi.com
Coatings Free FullText Electrodeposition of Copper Oxides as Cost Copper Oxide + H2 determine the formula of copper(ii) oxide by reducing it using hydrogen or methane, in one of three methods available to you in this practical. Cuo + h2 → cu + h2o. Copper (ii) oxide and hydrogen gas. Copper oxide hydrogen copper water. At higher temperatures the process is much faster. how to balance cuo + h2 = cu. Copper Oxide + H2.
From www.science-revision.co.uk
Extracting metals Copper Oxide + H2 Copper (ii) oxide and hydrogen gas. Natural gas (mainly methane) can also be used as a. Copper oxide hydrogen copper water. At higher temperatures the process is much faster. determine the formula of copper(ii) oxide by reducing it using hydrogen or methane, in one of three methods available to you in this practical. how to balance cuo +. Copper Oxide + H2.
From www.science-revision.co.uk
Displacement reactions Copper Oxide + H2 determine the formula of copper(ii) oxide by reducing it using hydrogen or methane, in one of three methods available to you in this practical. Natural gas (mainly methane) can also be used as a. Copper oxide hydrogen copper water. copper oxidizes slowly in air, corroding to produce a brown or green patina. how to balance cuo +. Copper Oxide + H2.
From www.sarthaks.com
When hydrogen gas is passed over heated copper (II) oxide, copper and Copper Oxide + H2 copper oxidizes slowly in air, corroding to produce a brown or green patina. determine the formula of copper(ii) oxide by reducing it using hydrogen or methane, in one of three methods available to you in this practical. the study of the reduction of copper oxide (cuo) by hydrogen (h 2) is helpful in elucidating the reduction mechanism. Copper Oxide + H2.
From www.youtube.com
Chemical Properties of Hydrogen Reaction of hydrogen with copper II Copper Oxide + H2 At higher temperatures the process is much faster. the study of the reduction of copper oxide (cuo) by hydrogen (h 2) is helpful in elucidating the reduction mechanism of oxygen carriers. cuo + h2 = cu2 + h2o is a single displacement (substitution) reaction where two moles of copper(ii) oxide [cuo] and. copper(ii) oxide can be reduced. Copper Oxide + H2.
From www.inoxia.co.uk
Buy Copper (II) Oxide at Inoxia Ltd Copper Oxide + H2 how to balance cuo + h2 = cu + h2o : copper oxidizes slowly in air, corroding to produce a brown or green patina. Here oxidation state of cu in cuo is +2 and that of h in h2 is 0. copper(ii) oxide can be reduced by hydrogen and its formula determined. determine the formula of. Copper Oxide + H2.
From www.youtube.com
Type of Reaction for Cu(OH)2 = CuO + H2O YouTube Copper Oxide + H2 the study of the reduction of copper oxide (cuo) by hydrogen (h 2) is helpful in elucidating the reduction mechanism of oxygen carriers. cuo + h2 = cu2 + h2o is a single displacement (substitution) reaction where two moles of copper(ii) oxide [cuo] and. how to balance cuo + h2 = cu + h2o : copper(ii). Copper Oxide + H2.
From www.mdpi.com
Catalysts Free FullText CopperCeriumTin Oxide Catalysts for Copper Oxide + H2 Here oxidation state of cu in cuo is +2 and that of h in h2 is 0. Copper oxide hydrogen copper water. Copper (ii) oxide and hydrogen gas. the study of the reduction of copper oxide (cuo) by hydrogen (h 2) is helpful in elucidating the reduction mechanism of oxygen carriers. how to balance cuo + h2 =. Copper Oxide + H2.
From study.com
Copper II Oxide Formula, Properties & Structure Lesson Copper Oxide + H2 copper(ii) oxide can be reduced by hydrogen and its formula determined. Cuo + h2 → cu + h2o. Copper oxide hydrogen copper water. cuo + h2 = cu2 + h2o is a single displacement (substitution) reaction where two moles of copper(ii) oxide [cuo] and. copper oxidizes slowly in air, corroding to produce a brown or green patina.. Copper Oxide + H2.
From www.youtube.com
Copper Oxide Synthesis YouTube Copper Oxide + H2 copper(ii) oxide can be reduced by hydrogen and its formula determined. Copper (ii) oxide and hydrogen gas. how to balance cuo + h2 = cu + h2o : Here oxidation state of cu in cuo is +2 and that of h in h2 is 0. Copper oxide hydrogen copper water. Natural gas (mainly methane) can also be used. Copper Oxide + H2.