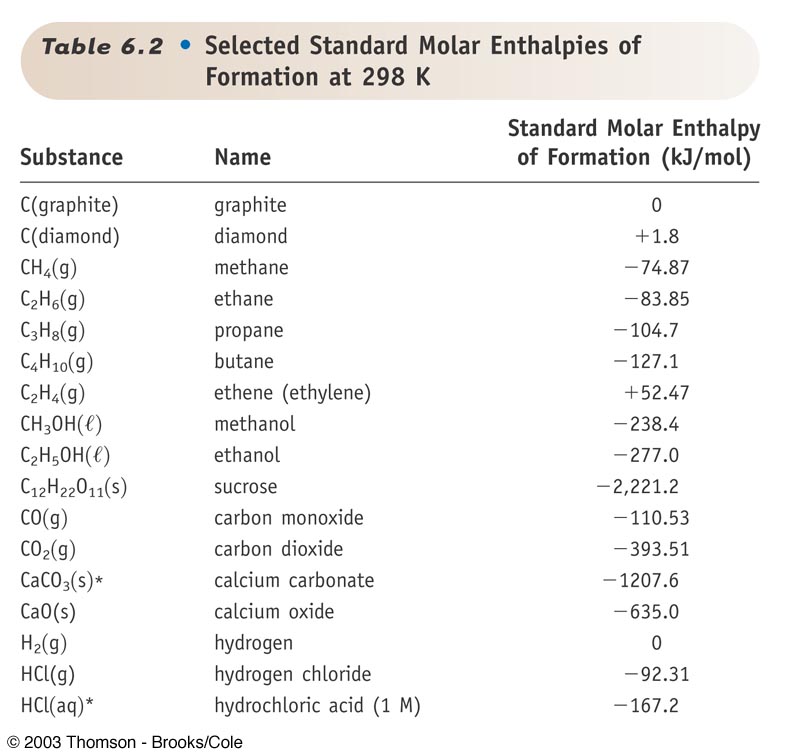
Standard Enthalpy Of Formation Magnesium Hydroxide . the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its. Magnesium hydroxide reacts with acids, forming the magnesium salt of said acid and water. Mg (oh) 2 + 2 hcl → mgcl 2 +. the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of.
from people.chem.umass.edu
193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its. Mg (oh) 2 + 2 hcl → mgcl 2 +. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. Magnesium hydroxide reacts with acids, forming the magnesium salt of said acid and water.
to Adobe GoLive 6
Standard Enthalpy Of Formation Magnesium Hydroxide the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. Magnesium hydroxide reacts with acids, forming the magnesium salt of said acid and water. Mg (oh) 2 + 2 hcl → mgcl 2 +. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its. the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under.
From dxogyycoh.blob.core.windows.net
Standard Enthalpy Of Formation Barium Hydroxide at Alice Connor blog Standard Enthalpy Of Formation Magnesium Hydroxide the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. Magnesium hydroxide reacts with acids, forming the magnesium salt of said acid and water. the enthalpy. Standard Enthalpy Of Formation Magnesium Hydroxide.
From momentumclubs.org
🎉 Heat of formation of magnesium oxide. Essay about Heat of formation Standard Enthalpy Of Formation Magnesium Hydroxide Mg (oh) 2 + 2 hcl → mgcl 2 +. Magnesium hydroxide reacts with acids, forming the magnesium salt of said acid and water. the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its. the standard enthalpy of formation is defined as the change in enthalpy when one mole of. Standard Enthalpy Of Formation Magnesium Hydroxide.
From www.numerade.com
SOLVED Calculate the standard enthalpy of combustion for propane gas Standard Enthalpy Of Formation Magnesium Hydroxide the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Mg (oh) 2 + 2 hcl → mgcl 2 +. Magnesium hydroxide reacts with acids, forming the magnesium salt of said acid and. Standard Enthalpy Of Formation Magnesium Hydroxide.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Magnesium Hydroxide Mg (oh) 2 + 2 hcl → mgcl 2 +. the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its. the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. 193 rows in chemistry and thermodynamics,. Standard Enthalpy Of Formation Magnesium Hydroxide.
From studylib.net
Lab XIV Determing the heat of formation of MgO Standard Enthalpy Of Formation Magnesium Hydroxide the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered. Standard Enthalpy Of Formation Magnesium Hydroxide.
From brainly.in
. At 298 K the standard enthalpies of formation ofH2O(l) and H,0,(l Standard Enthalpy Of Formation Magnesium Hydroxide 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its. Mg (oh) 2. Standard Enthalpy Of Formation Magnesium Hydroxide.
From www.researchgate.net
Standard Gibbs free energy of formation, standard enthalpy of formation Standard Enthalpy Of Formation Magnesium Hydroxide the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its. Mg (oh) 2 + 2 hcl → mgcl 2 +. the table below lists the standard molar. Standard Enthalpy Of Formation Magnesium Hydroxide.
From www.researchgate.net
2. Standard enthalpy of formation of βCu 2x Se(s) at 298.15 K Standard Enthalpy Of Formation Magnesium Hydroxide the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. the enthalpy of formation (δhf) is the enthalpy change that accompanies the. Standard Enthalpy Of Formation Magnesium Hydroxide.
From www.transtutors.com
(Solved) EXPERIMENT 13 THERMODYNAMICS ENTHALPY OF FORMATION Standard Enthalpy Of Formation Magnesium Hydroxide 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. Magnesium hydroxide reacts with acids, forming the magnesium salt of said acid and water. Mg (oh) 2 +. Standard Enthalpy Of Formation Magnesium Hydroxide.
From www.chegg.com
Solved Calculate the enthalpy of formation for magnesium Standard Enthalpy Of Formation Magnesium Hydroxide 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. Mg (oh) 2 + 2 hcl → mgcl 2 +. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. the enthalpy of formation (δhf) is. Standard Enthalpy Of Formation Magnesium Hydroxide.
From dxouglhlh.blob.core.windows.net
Standard Enthalpy Of Formation Mg at David Leon blog Standard Enthalpy Of Formation Magnesium Hydroxide the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered. Standard Enthalpy Of Formation Magnesium Hydroxide.
From www.numerade.com
SOLVED 5) How many moles of magnesium were used to create this amount Standard Enthalpy Of Formation Magnesium Hydroxide the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. Magnesium hydroxide reacts with acids, forming the magnesium salt of said acid and water. the table below. Standard Enthalpy Of Formation Magnesium Hydroxide.
From www.numerade.com
SOLVED Solid magnesium hydroxide into gaseous water and Standard Enthalpy Of Formation Magnesium Hydroxide the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. Mg (oh) 2 + 2 hcl → mgcl 2 +. Magnesium hydroxide reacts with acids, forming the magnesium salt of said acid and water. 136 rows standard enthalpy change of formation (data table) these tables. Standard Enthalpy Of Formation Magnesium Hydroxide.
From www.numerade.com
SOLVED4 The standard enthalpy of formation of magnesium oxide_ Use Standard Enthalpy Of Formation Magnesium Hydroxide 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard. Standard Enthalpy Of Formation Magnesium Hydroxide.
From tateho-chemical.com
Magnesium Hydroxide Based Heat Storage Material CHARGEMAG® Standard Enthalpy Of Formation Magnesium Hydroxide the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its. the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. the standard enthalpy of formation is defined as the change in enthalpy when one mole of. Standard Enthalpy Of Formation Magnesium Hydroxide.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation of reaction 2H2(g Standard Enthalpy Of Formation Magnesium Hydroxide the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. Magnesium hydroxide reacts with acids, forming the magnesium salt of said acid and water. the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its. 193 rows. Standard Enthalpy Of Formation Magnesium Hydroxide.
From www.myxxgirl.com
Standard Enthalpy Formation Table My XXX Hot Girl Standard Enthalpy Of Formation Magnesium Hydroxide Mg (oh) 2 + 2 hcl → mgcl 2 +. Magnesium hydroxide reacts with acids, forming the magnesium salt of said acid and water. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f. Standard Enthalpy Of Formation Magnesium Hydroxide.
From people.chem.umass.edu
to Adobe GoLive 6 Standard Enthalpy Of Formation Magnesium Hydroxide the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. Magnesium hydroxide reacts with acids, forming the magnesium salt of said acid and water. the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured. Standard Enthalpy Of Formation Magnesium Hydroxide.
From studylib.net
I. Standard Enthalpies of Formation Standard Enthalpy Of Formation Magnesium Hydroxide 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard. Standard Enthalpy Of Formation Magnesium Hydroxide.
From dxouglhlh.blob.core.windows.net
Standard Enthalpy Of Formation Mg at David Leon blog Standard Enthalpy Of Formation Magnesium Hydroxide 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. Mg (oh) 2 + 2 hcl → mgcl 2 +. the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. 136 rows standard enthalpy change of formation. Standard Enthalpy Of Formation Magnesium Hydroxide.
From www.numerade.com
SOLVED 4. Use standard enthalpies of formation to calculate the Standard Enthalpy Of Formation Magnesium Hydroxide the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its. the standard enthalpy of formation is defined as the change in enthalpy when one mole of. Standard Enthalpy Of Formation Magnesium Hydroxide.
From www.youtube.com
Thermochemistry Enthalpies of Formation YouTube Standard Enthalpy Of Formation Magnesium Hydroxide the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. Magnesium hydroxide reacts with acids, forming the magnesium salt of said acid and. Standard Enthalpy Of Formation Magnesium Hydroxide.
From dxogyycoh.blob.core.windows.net
Standard Enthalpy Of Formation Barium Hydroxide at Alice Connor blog Standard Enthalpy Of Formation Magnesium Hydroxide Mg (oh) 2 + 2 hcl → mgcl 2 +. the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its. the standard enthalpy of formation is. Standard Enthalpy Of Formation Magnesium Hydroxide.
From www.chegg.com
Solved Using the standard enthalpies of formation listed in Standard Enthalpy Of Formation Magnesium Hydroxide the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. Mg (oh) 2 + 2 hcl → mgcl 2 +. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. Magnesium hydroxide reacts. Standard Enthalpy Of Formation Magnesium Hydroxide.
From www.numerade.com
SOLVED Consider the oxidationreduction reaction Mg(s) + H2O(l) â Standard Enthalpy Of Formation Magnesium Hydroxide the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. Magnesium hydroxide reacts with acids, forming the magnesium salt of said acid and water. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. the enthalpy of formation. Standard Enthalpy Of Formation Magnesium Hydroxide.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Magnesium Hydroxide 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a. Standard Enthalpy Of Formation Magnesium Hydroxide.
From studylib.net
Experiment 9 Enthalpy of Formation of Magnesium Oxide Standard Enthalpy Of Formation Magnesium Hydroxide the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of. Standard Enthalpy Of Formation Magnesium Hydroxide.
From talisman-intl.com
🎉 Enthalpy of formation of mgo. Enthalpy of formation of magnesium Standard Enthalpy Of Formation Magnesium Hydroxide Magnesium hydroxide reacts with acids, forming the magnesium salt of said acid and water. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. 136 rows standard enthalpy. Standard Enthalpy Of Formation Magnesium Hydroxide.
From keplarllp.com
😎 Enthalpy of mgo. What is the standard change in enthalpy of formation Standard Enthalpy Of Formation Magnesium Hydroxide the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. Magnesium hydroxide reacts with acids, forming the magnesium salt of said acid and water. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. Mg (oh) 2. Standard Enthalpy Of Formation Magnesium Hydroxide.
From www.numerade.com
Consider the following reaction 2 H2S (g) + 3 O2 (g) → 2 SO2 (g) + 2 Standard Enthalpy Of Formation Magnesium Hydroxide the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation. Standard Enthalpy Of Formation Magnesium Hydroxide.
From www.numerade.com
SOLVED a) The enthalpy change for the reaction in which solid Standard Enthalpy Of Formation Magnesium Hydroxide Magnesium hydroxide reacts with acids, forming the magnesium salt of said acid and water. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its. Mg (oh) 2 + 2 hcl →. Standard Enthalpy Of Formation Magnesium Hydroxide.
From mavink.com
Standard Enthalpy Chart Standard Enthalpy Of Formation Magnesium Hydroxide 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of. 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard. Standard Enthalpy Of Formation Magnesium Hydroxide.
From dxouglhlh.blob.core.windows.net
Standard Enthalpy Of Formation Mg at David Leon blog Standard Enthalpy Of Formation Magnesium Hydroxide 136 rows standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a. Mg (oh) 2 + 2 hcl → mgcl 2 +. the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. the standard enthalpy of formation. Standard Enthalpy Of Formation Magnesium Hydroxide.
From www.vrogue.co
Standard Enthalpy Of Formation And Standard Free Ener vrogue.co Standard Enthalpy Of Formation Magnesium Hydroxide the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. the standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state. Magnesium hydroxide reacts with acids, forming the magnesium salt of said acid and. Standard Enthalpy Of Formation Magnesium Hydroxide.
From brandonkss.github.io
Enthalpy Of Formation Chart Standard Enthalpy Of Formation Magnesium Hydroxide the table below lists the standard molar enthalpy of formation (δ h ∘ f δ h f ∘) of various substances, measured under. the enthalpy of formation (δhf) is the enthalpy change that accompanies the formation of a compound from its. Mg (oh) 2 + 2 hcl → mgcl 2 +. 136 rows standard enthalpy change of. Standard Enthalpy Of Formation Magnesium Hydroxide.