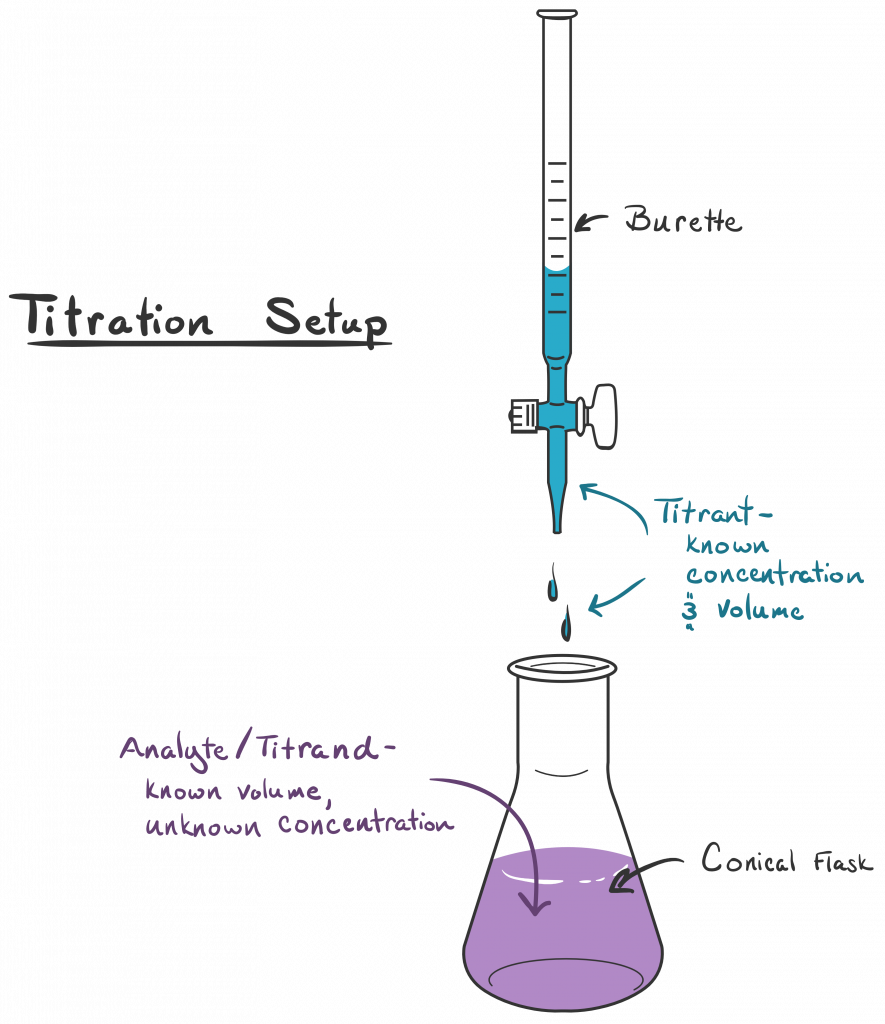
Titration In Assay . A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. The video below demonstrates the titration when small, measured amounts of a known permaganate solution are added. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. At the endpoint, the number of moles of. A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being. For example, you can use it to find the concentration. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent.
from www.microlit.com
Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. At the endpoint, the number of moles of. For example, you can use it to find the concentration. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. The video below demonstrates the titration when small, measured amounts of a known permaganate solution are added. A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.
An Advanced Guide to Titration Microlit
Titration In Assay The video below demonstrates the titration when small, measured amounts of a known permaganate solution are added. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The video below demonstrates the titration when small, measured amounts of a known permaganate solution are added. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. At the endpoint, the number of moles of. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. For example, you can use it to find the concentration. A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the.
From www.showme.com
Titration calculation Science, Chemistry, Physical Chemistry ShowMe Titration In Assay A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the. Titration In Assay.
From www.microlit.com
An Advanced Guide to Titration Microlit Titration In Assay Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. For example, you can use it to find the concentration. At the endpoint, the number of moles. Titration In Assay.
From letitsnowglobe.co.uk
Titration procedure pdf Titration In Assay Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively. Titration In Assay.
From www.scienceabc.com
Titration Chemistry Definition, Explanation, Formula And Calculation Titration In Assay Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. For example, you can use it to find the concentration. Titration is a way of measuring. Titration In Assay.
From www.vrogue.co
Strong Acid Weak Base Titrations vrogue.co Titration In Assay For example, you can use it to find the concentration. A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being. At the endpoint, the number of moles of. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. A titration is the process. Titration In Assay.
From www.youtube.com
Assay of Ibuprofen IP Acid Base Titration Pharm Analysis Titration In Assay Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being. At the endpoint, the number of moles of. Titration is the slow addition of one solution of a known. Titration In Assay.
From www.coriolis-pharma.com
Virus titration / TCID50 assay Coriolis Pharma Titration In Assay The video below demonstrates the titration when small, measured amounts of a known permaganate solution are added. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. At the endpoint, the. Titration In Assay.
From pubs.rsc.org
A convenient chemical titration method to measure M( ii )/M( iii Titration In Assay Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample. Titration In Assay.
From www.researchgate.net
The TCID50 assay. A Schematic of a 96well viral titration plate. An Titration In Assay The video below demonstrates the titration when small, measured amounts of a known permaganate solution are added. A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being. For example, you can use it to find the concentration. A titration is the process of determining the quantity of one substance by adding measured. Titration In Assay.
From www.pharmaspecialists.com
Potentiometric Titration in Pharmaceutical Analysis Titration In Assay Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a way of measuring the concentration of something, usually the concentration. Titration In Assay.
From theedge.com.hk
Chemistry How To Titration The Edge Titration In Assay Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A titration is the process of determining the quantity of one substance by adding measured amounts of. Titration In Assay.
From www.alamy.com
Titration, titrimetry or volumetric analysis. A burette and Erlenmeyer Titration In Assay Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. For example, you can use it to find the concentration. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. The video below demonstrates the titration when small,. Titration In Assay.
From www.studocu.com
Hydrogen Peroxide Assay ASSAY OF HYDROGEN PEROXIDE BY. REDUCTION Titration In Assay A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume. Titration In Assay.
From www.youtube.com
Titration Experiment & Calculate the Molarity of Acetic Acid in Vinegar Titration In Assay Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being. The video below demonstrates the titration when small, measured amounts of a known permaganate solution are added. For example, you can use it. Titration In Assay.
From www.researchgate.net
Representative assay titration curve illustrating equivalence Titration In Assay Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. The basic process involves adding a standard solution of one reagent to a known amount of the. Titration In Assay.
From www.tutormyself.com
233 (Triple only) describe how to carry out an acidalkali titration Titration In Assay At the endpoint, the number of moles of. A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being. For example, you can use it to find the concentration. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. Titration. Titration In Assay.
From www.youtube.com
Assay of Aspirin and Ibuprofen as per IP Concept of Direct titration Titration In Assay A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. At the endpoint, the number of moles of. A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being. The video below demonstrates the titration when small, measured amounts of a known. Titration In Assay.
From www.slideserve.com
PPT Ch 6 Good Titrations PowerPoint Presentation, free download ID Titration In Assay The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by. Titration In Assay.
From www.researchgate.net
Titration of assay reagents. A) AF488MCP1 was serially diluted in Titration In Assay Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. For example, you can use it to find the concentration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. A titration is a laboratory technique used. Titration In Assay.
From letitsnowglobe.co.uk
Titration procedure pdf Titration In Assay A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. At the. Titration In Assay.
From chem.libretexts.org
9.3 Complexation Titrations Chemistry LibreTexts Titration In Assay Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being. For example, you. Titration In Assay.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration In Assay For example, you can use it to find the concentration. The video below demonstrates the titration when small, measured amounts of a known permaganate solution are added. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. A titration is a laboratory technique used to precisely measure molar concentration. Titration In Assay.
From letitsnowglobe.co.uk
Titration procedure pdf Titration In Assay A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The video below demonstrates the titration when small, measured amounts of a known permaganate solution are added. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the.. Titration In Assay.
From www.researchgate.net
Titration of antibodies in the agglutination assay A Eighteen plasma Titration In Assay Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. The video below demonstrates the titration when small, measured amounts of a known permaganate solution are. Titration In Assay.
From www.science-revision.co.uk
Titrations Titration In Assay A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. For example, you can use it to find the concentration. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. At the endpoint, the number of. Titration In Assay.
From www.slideserve.com
PPT Neutralization Reactions using Titration Method PowerPoint Titration In Assay Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. At the endpoint, the number of moles of. For example, you can use it to find the concentration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known. Titration In Assay.
From chem.libretexts.org
AcidBase Titrations Chemistry LibreTexts Titration In Assay For example, you can use it to find the concentration. Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. At the endpoint, the number of moles of. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. Titration, process of. Titration In Assay.
From www.pharmafood.org
Assay Test Procedure for Titanium Dioxide (TIO2) by Titration Method. Titration In Assay Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. At the endpoint, the number of moles of. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. The video below demonstrates the titration when small, measured. Titration In Assay.
From pubs.rsc.org
A convenient chemical titration method to measure M( ii )/M( iii Titration In Assay The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. Titration is a way of measuring the concentration of something, usually the concentration of a substance. Titration In Assay.
From mungfali.com
Acid Base Titration Procedure Titration In Assay At the endpoint, the number of moles of. For example, you can use it to find the concentration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being. A titration is the. Titration In Assay.
From www.researchgate.net
SNAPbased TRFRET titration assay. (A) Scheme of the titration binding Titration In Assay A quantitative chemical analysis in which a defined amount of titrant reacts quantitatively with the sample compound being. For example, you can use it to find the concentration. Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. At the endpoint, the number of moles of.. Titration In Assay.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Titration In Assay A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. For example, you can use it to find the concentration. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution. The video below demonstrates the titration when small, measured. Titration In Assay.
From www.youtube.com
Experiment 10 Assay Determination of Aspirin using Back Titration Titration In Assay Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. A titration is the process of determining the quantity of one substance by adding measured amounts of another substance, called the. A titration is a laboratory technique used to precisely measure molar concentration of an unknown solution using a known solution.. Titration In Assay.
From slidetodoc.com
Titration Colour Changes SLSS Science Limerick Education Centre Titration In Assay Titration is the slow addition of one solution of a known concentration (called a titrant) to a known volume of another solution of unknown. For example, you can use it to find the concentration. Titration, process of chemical analysis in which the quantity of some constituent of a sample is determined by the gradual addition to the. The basic process. Titration In Assay.
From chem.libretexts.org
Quantitative Analysis Nuts and Bolts Chemistry LibreTexts Titration In Assay Titration is a way of measuring the concentration of something, usually the concentration of a substance in a solution. At the endpoint, the number of moles of. The basic process involves adding a standard solution of one reagent to a known amount of the unknown solution of a different reagent. A titration is a laboratory technique used to precisely measure. Titration In Assay.