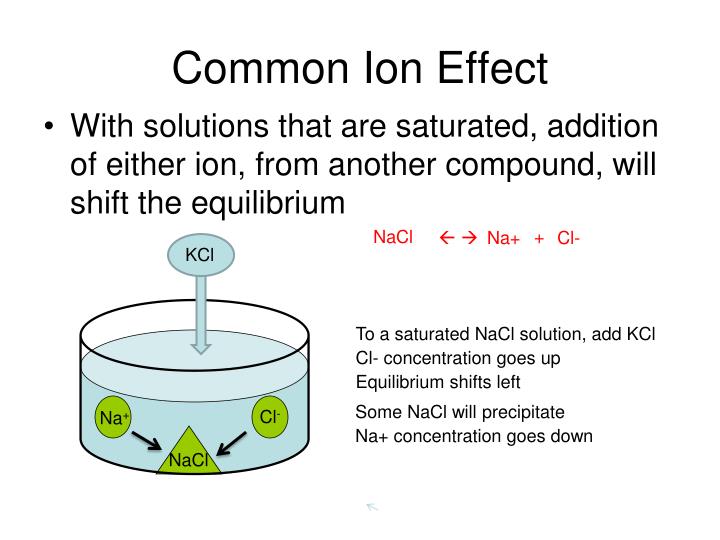
Buffers Common Ion Effect . Either weak acids and their conjugate bases or weak. • they behave as buffers by. The common ion effect is when a given ion is added to a mixture at equilibrium that already contains the given ion. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in. A buffer works through a phenomenon called the common ion effect. The common ion effect occurs when a given ion is. • combination of a weak acid or base and a conjugate salt. Buffers are solutions which resist changes in ph. The sulfate ion concentration remains the same as it is a common ion and its concentration is the same in both solutions.
from www.slideserve.com
The sulfate ion concentration remains the same as it is a common ion and its concentration is the same in both solutions. Buffers are solutions which resist changes in ph. The common ion effect is when a given ion is added to a mixture at equilibrium that already contains the given ion. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in. The common ion effect occurs when a given ion is. • combination of a weak acid or base and a conjugate salt. Either weak acids and their conjugate bases or weak. A buffer works through a phenomenon called the common ion effect. • they behave as buffers by.
PPT Ksp and the Common Ion Effect PowerPoint Presentation ID5978246
Buffers Common Ion Effect The common ion effect occurs when a given ion is. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in. The sulfate ion concentration remains the same as it is a common ion and its concentration is the same in both solutions. A buffer works through a phenomenon called the common ion effect. Buffers are solutions which resist changes in ph. Either weak acids and their conjugate bases or weak. • they behave as buffers by. The common ion effect occurs when a given ion is. The common ion effect is when a given ion is added to a mixture at equilibrium that already contains the given ion. • combination of a weak acid or base and a conjugate salt.
From animalia-life.club
Phosphate Buffer System Equation Buffers Common Ion Effect Buffers are solutions which resist changes in ph. • combination of a weak acid or base and a conjugate salt. Either weak acids and their conjugate bases or weak. • they behave as buffers by. The sulfate ion concentration remains the same as it is a common ion and its concentration is the same in both solutions. The common ion. Buffers Common Ion Effect.
From slidetodoc.com
Common Ion Effect Buffers Common Ion Effect l Buffers Common Ion Effect Buffers are solutions which resist changes in ph. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in. • they behave as buffers by. Either weak acids and their conjugate bases or weak. The sulfate ion concentration remains the same as it is a common ion and its. Buffers Common Ion Effect.
From slidetodoc.com
Common Ion Effect Buffers Common Ion Effect l Buffers Common Ion Effect A buffer works through a phenomenon called the common ion effect. • combination of a weak acid or base and a conjugate salt. Either weak acids and their conjugate bases or weak. Buffers are solutions which resist changes in ph. The sulfate ion concentration remains the same as it is a common ion and its concentration is the same in. Buffers Common Ion Effect.
From www.slideserve.com
PPT Unit 18 AcidBase Equilibria Buffers & Hydrolysis PowerPoint Buffers Common Ion Effect The sulfate ion concentration remains the same as it is a common ion and its concentration is the same in both solutions. The common ion effect is when a given ion is added to a mixture at equilibrium that already contains the given ion. Buffers are solutions which resist changes in ph. A buffer works through a phenomenon called the. Buffers Common Ion Effect.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID3308656 Buffers Common Ion Effect A buffer works through a phenomenon called the common ion effect. Either weak acids and their conjugate bases or weak. The sulfate ion concentration remains the same as it is a common ion and its concentration is the same in both solutions. • combination of a weak acid or base and a conjugate salt. • they behave as buffers by.. Buffers Common Ion Effect.
From slidetodoc.com
Common Ion Effect Buffers Common Ion Effect l Buffers Common Ion Effect A buffer works through a phenomenon called the common ion effect. • combination of a weak acid or base and a conjugate salt. The common ion effect occurs when a given ion is. Either weak acids and their conjugate bases or weak. The sulfate ion concentration remains the same as it is a common ion and its concentration is the. Buffers Common Ion Effect.
From slideplayer.com
Ionic Equilibria Part II Buffers and Titration Curves ppt download Buffers Common Ion Effect The sulfate ion concentration remains the same as it is a common ion and its concentration is the same in both solutions. Buffers are solutions which resist changes in ph. • they behave as buffers by. • combination of a weak acid or base and a conjugate salt. Either weak acids and their conjugate bases or weak. A buffer works. Buffers Common Ion Effect.
From slidetodoc.com
Common Ion Effect Buffers Common Ion Effect l Buffers Common Ion Effect The common ion effect occurs when a given ion is. The common ion effect is when a given ion is added to a mixture at equilibrium that already contains the given ion. Buffers are solutions which resist changes in ph. • they behave as buffers by. The common ion effect describes the effect on equilibrium that occurs when a common. Buffers Common Ion Effect.
From general.chemistrysteps.com
The Effect of a Common Ion on Solubility Chemistry Steps Buffers Common Ion Effect Buffers are solutions which resist changes in ph. The common ion effect is when a given ion is added to a mixture at equilibrium that already contains the given ion. Either weak acids and their conjugate bases or weak. • combination of a weak acid or base and a conjugate salt. The common ion effect occurs when a given ion. Buffers Common Ion Effect.
From slidetodoc.com
Common Ion Effect Buffers Common Ion Effect Sometimes Buffers Common Ion Effect The common ion effect is when a given ion is added to a mixture at equilibrium that already contains the given ion. A buffer works through a phenomenon called the common ion effect. Buffers are solutions which resist changes in ph. • they behave as buffers by. The common ion effect occurs when a given ion is. The sulfate ion. Buffers Common Ion Effect.
From www.youtube.com
Common Ion Effect and Buffer Solutions YouTube Buffers Common Ion Effect The common ion effect is when a given ion is added to a mixture at equilibrium that already contains the given ion. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in. A buffer works through a phenomenon called the common ion effect. Buffers are solutions which resist. Buffers Common Ion Effect.
From slideplayer.com
Ionic Equilibria Part II Buffers and Titration Curves ppt download Buffers Common Ion Effect A buffer works through a phenomenon called the common ion effect. • combination of a weak acid or base and a conjugate salt. The sulfate ion concentration remains the same as it is a common ion and its concentration is the same in both solutions. Either weak acids and their conjugate bases or weak. The common ion effect is when. Buffers Common Ion Effect.
From www.studocu.com
Buffers notes BUFFER common ion effect The CommonIon Effect Buffers Common Ion Effect The common ion effect is when a given ion is added to a mixture at equilibrium that already contains the given ion. A buffer works through a phenomenon called the common ion effect. Buffers are solutions which resist changes in ph. • they behave as buffers by. Either weak acids and their conjugate bases or weak. The common ion effect. Buffers Common Ion Effect.
From www.studocu.com
Chap 18 pt1 Common Ion Effect and Buffers Chapter 18 AcidBase Buffers Common Ion Effect Either weak acids and their conjugate bases or weak. Buffers are solutions which resist changes in ph. The sulfate ion concentration remains the same as it is a common ion and its concentration is the same in both solutions. A buffer works through a phenomenon called the common ion effect. The common ion effect is when a given ion is. Buffers Common Ion Effect.
From www.youtube.com
Common Ion Effect // HSC Chemistry YouTube Buffers Common Ion Effect • they behave as buffers by. Buffers are solutions which resist changes in ph. • combination of a weak acid or base and a conjugate salt. A buffer works through a phenomenon called the common ion effect. Either weak acids and their conjugate bases or weak. The common ion effect occurs when a given ion is. The common ion effect. Buffers Common Ion Effect.
From www.slideserve.com
PPT COMMON ION EFFECT PowerPoint Presentation, free download ID6209182 Buffers Common Ion Effect The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in. The sulfate ion concentration remains the same as it is a common ion and its concentration is the same in both solutions. The common ion effect occurs when a given ion is. • combination of a weak acid. Buffers Common Ion Effect.
From slidetodoc.com
Common Ion Effect Buffers Common Ion Effect Sometimes Buffers Common Ion Effect The common ion effect is when a given ion is added to a mixture at equilibrium that already contains the given ion. • they behave as buffers by. The common ion effect occurs when a given ion is. Buffers are solutions which resist changes in ph. • combination of a weak acid or base and a conjugate salt. A buffer. Buffers Common Ion Effect.
From classnotes.org.in
Buffer solution and Buffer Action Chemistry, Class 11, Ionic Equilibrium Buffers Common Ion Effect Buffers are solutions which resist changes in ph. A buffer works through a phenomenon called the common ion effect. • they behave as buffers by. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in. The sulfate ion concentration remains the same as it is a common ion. Buffers Common Ion Effect.
From dokumen.tips
(PPT) Unit 6 Chpt 15 Acid/Base Equilibria Common Ion Effect Buffers Buffers Common Ion Effect • combination of a weak acid or base and a conjugate salt. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in. Either weak acids and their conjugate bases or weak. The common ion effect occurs when a given ion is. A buffer works through a phenomenon called. Buffers Common Ion Effect.
From dokumen.tips
(PPT) Common Ion Effect Buffers. Common Ion Effect Sometimes the Buffers Common Ion Effect Either weak acids and their conjugate bases or weak. A buffer works through a phenomenon called the common ion effect. The sulfate ion concentration remains the same as it is a common ion and its concentration is the same in both solutions. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that. Buffers Common Ion Effect.
From slidetodoc.com
Common Ion Effect Buffers Common Ion Effect l Buffers Common Ion Effect • combination of a weak acid or base and a conjugate salt. The common ion effect is when a given ion is added to a mixture at equilibrium that already contains the given ion. The sulfate ion concentration remains the same as it is a common ion and its concentration is the same in both solutions. The common ion effect. Buffers Common Ion Effect.
From slidetodoc.com
Common Ion Effect and Buffers Ch 17 in Buffers Common Ion Effect Buffers are solutions which resist changes in ph. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in. The common ion effect is when a given ion is added to a mixture at equilibrium that already contains the given ion. A buffer works through a phenomenon called the. Buffers Common Ion Effect.
From slidetodoc.com
Common Ion Effect Buffers Common Ion Effect l Buffers Common Ion Effect A buffer works through a phenomenon called the common ion effect. The common ion effect is when a given ion is added to a mixture at equilibrium that already contains the given ion. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in. Either weak acids and their. Buffers Common Ion Effect.
From pdfslide.net
(PPT) Buffers, Titrations, and Aqueous Equilibria. Common Ion Effect Buffers Common Ion Effect The common ion effect is when a given ion is added to a mixture at equilibrium that already contains the given ion. • combination of a weak acid or base and a conjugate salt. • they behave as buffers by. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already. Buffers Common Ion Effect.
From slideplayer.com
Buffers Made Easy. Common ion effect Common Ion the presence of an ion Buffers Common Ion Effect • combination of a weak acid or base and a conjugate salt. The common ion effect occurs when a given ion is. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in. A buffer works through a phenomenon called the common ion effect. The common ion effect is. Buffers Common Ion Effect.
From slideplayer.com
Ionic Equilibria Part II Buffers and Titration Curves ppt download Buffers Common Ion Effect A buffer works through a phenomenon called the common ion effect. • combination of a weak acid or base and a conjugate salt. Either weak acids and their conjugate bases or weak. Buffers are solutions which resist changes in ph. The sulfate ion concentration remains the same as it is a common ion and its concentration is the same in. Buffers Common Ion Effect.
From slidetodoc.com
Common Ion Effect Buffers Common Ion Effect Sometimes Buffers Common Ion Effect The common ion effect occurs when a given ion is. The sulfate ion concentration remains the same as it is a common ion and its concentration is the same in both solutions. Either weak acids and their conjugate bases or weak. A buffer works through a phenomenon called the common ion effect. The common ion effect is when a given. Buffers Common Ion Effect.
From slideplayer.com
Ionic Equilibria Part II Buffers and Titration Curves ppt download Buffers Common Ion Effect • they behave as buffers by. A buffer works through a phenomenon called the common ion effect. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in. The common ion effect is when a given ion is added to a mixture at equilibrium that already contains the given. Buffers Common Ion Effect.
From www.slideserve.com
PPT Chapter 17a PowerPoint Presentation, free download ID4339402 Buffers Common Ion Effect • combination of a weak acid or base and a conjugate salt. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in. The common ion effect is when a given ion is added to a mixture at equilibrium that already contains the given ion. The common ion effect. Buffers Common Ion Effect.
From www.slideserve.com
PPT Ksp and the Common Ion Effect PowerPoint Presentation ID5978246 Buffers Common Ion Effect The common ion effect occurs when a given ion is. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in. The common ion effect is when a given ion is added to a mixture at equilibrium that already contains the given ion. The sulfate ion concentration remains the. Buffers Common Ion Effect.
From slideplayer.com
Ionic Equilibria Part II Buffers and Titration Curves ppt download Buffers Common Ion Effect The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in. • they behave as buffers by. Either weak acids and their conjugate bases or weak. • combination of a weak acid or base and a conjugate salt. A buffer works through a phenomenon called the common ion effect.. Buffers Common Ion Effect.
From www.slideserve.com
PPT Chapter 16 Introduction to Buffers PowerPoint Presentation, free Buffers Common Ion Effect The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in. • combination of a weak acid or base and a conjugate salt. Either weak acids and their conjugate bases or weak. The common ion effect is when a given ion is added to a mixture at equilibrium that. Buffers Common Ion Effect.
From www.slideserve.com
PPT Common Ion Effect PowerPoint Presentation, free download ID3469534 Buffers Common Ion Effect A buffer works through a phenomenon called the common ion effect. Buffers are solutions which resist changes in ph. The sulfate ion concentration remains the same as it is a common ion and its concentration is the same in both solutions. Either weak acids and their conjugate bases or weak. • combination of a weak acid or base and a. Buffers Common Ion Effect.
From slidetodoc.com
Common Ion Effect Buffers Common Ion Effect l Buffers Common Ion Effect Buffers are solutions which resist changes in ph. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in. The sulfate ion concentration remains the same as it is a common ion and its concentration is the same in both solutions. A buffer works through a phenomenon called the. Buffers Common Ion Effect.
From slideplayer.com
Ionic Equilibria Part II Buffers and Titration Curves ppt download Buffers Common Ion Effect The common ion effect occurs when a given ion is. The common ion effect describes the effect on equilibrium that occurs when a common ion (an ion that is already contained in. Buffers are solutions which resist changes in ph. A buffer works through a phenomenon called the common ion effect. Either weak acids and their conjugate bases or weak.. Buffers Common Ion Effect.