Magnesium Hydroxide In Water Chemical Equation . It occurs in nature as the mineral brucite. What is the balanced chemical equation that describes the dissociation of the strong base magnesium hydroxide, mg (oh)2 in. Magnesium hydroxide can be prepared using a simple chemical reaction involving magnesium chloride (mgcl₂) and sodium hydroxide (naoh). Thermodynamics of the reaction can be. It is also called milk of magnesia or magnesium (2+). Magnesium hydroxide is an inorganic compound with the chemical formula mg (oh) 2. The balanced equation is mg (oh)₂ (s) ⇌ mg²⁺ (aq) + 2oh⁻ (aq) magnesium hydroxide is sparingly soluble in water. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. Calculate the net ionic equation for mg (oh)2 (s) + 2hcl (aq) = mgcl2 (aq) + 2h2o (l). It is a white solid. Magnesium hydroxide is an inorganic compound which has a low solubility in water.
from www.numerade.com
In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. Magnesium hydroxide is an inorganic compound which has a low solubility in water. The balanced equation is mg (oh)₂ (s) ⇌ mg²⁺ (aq) + 2oh⁻ (aq) magnesium hydroxide is sparingly soluble in water. Calculate the net ionic equation for mg (oh)2 (s) + 2hcl (aq) = mgcl2 (aq) + 2h2o (l). Magnesium hydroxide is an inorganic compound with the chemical formula mg (oh) 2. It occurs in nature as the mineral brucite. What is the balanced chemical equation that describes the dissociation of the strong base magnesium hydroxide, mg (oh)2 in. It is also called milk of magnesia or magnesium (2+). Magnesium hydroxide can be prepared using a simple chemical reaction involving magnesium chloride (mgcl₂) and sodium hydroxide (naoh). Thermodynamics of the reaction can be.
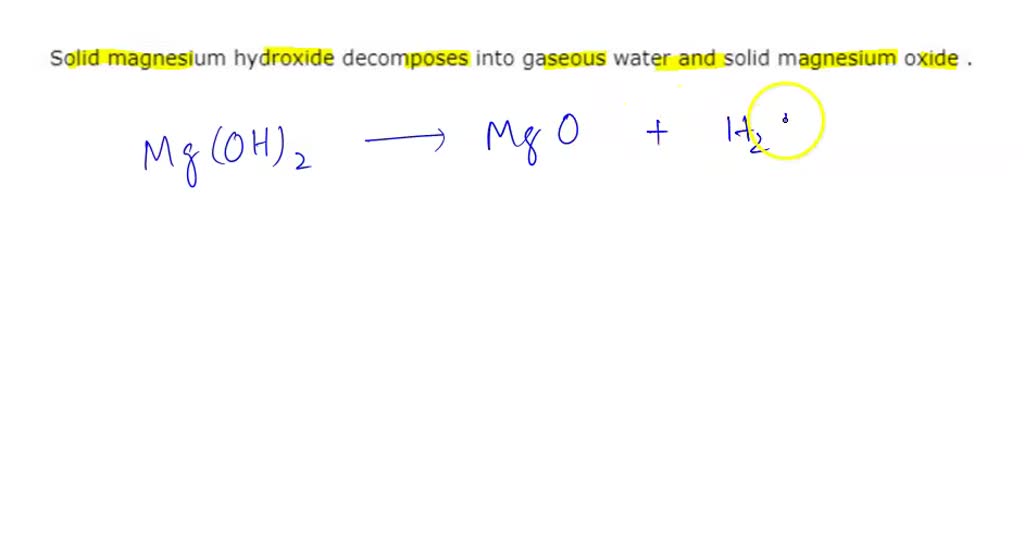
SOLVED Solid magnesium hydroxide into gaseous water and solid magnesium oxide Write
Magnesium Hydroxide In Water Chemical Equation Magnesium hydroxide is an inorganic compound which has a low solubility in water. It is also called milk of magnesia or magnesium (2+). Thermodynamics of the reaction can be. What is the balanced chemical equation that describes the dissociation of the strong base magnesium hydroxide, mg (oh)2 in. Magnesium hydroxide is an inorganic compound with the chemical formula mg (oh) 2. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. Magnesium hydroxide can be prepared using a simple chemical reaction involving magnesium chloride (mgcl₂) and sodium hydroxide (naoh). The balanced equation is mg (oh)₂ (s) ⇌ mg²⁺ (aq) + 2oh⁻ (aq) magnesium hydroxide is sparingly soluble in water. Calculate the net ionic equation for mg (oh)2 (s) + 2hcl (aq) = mgcl2 (aq) + 2h2o (l). It is a white solid. Magnesium hydroxide is an inorganic compound which has a low solubility in water. It occurs in nature as the mineral brucite.
From www.chegg.com
Solved Calculate the molar solubility of magnesium hydroxide Magnesium Hydroxide In Water Chemical Equation Calculate the net ionic equation for mg (oh)2 (s) + 2hcl (aq) = mgcl2 (aq) + 2h2o (l). In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. The balanced equation is mg (oh)₂ (s) ⇌ mg²⁺ (aq) + 2oh⁻ (aq) magnesium hydroxide is sparingly soluble in water. Magnesium hydroxide. Magnesium Hydroxide In Water Chemical Equation.
From www.numerade.com
SOLVED What will cause more Magnesium to be dissolved in a solution of Magnesium Hydroxide in Magnesium Hydroxide In Water Chemical Equation It is a white solid. Magnesium hydroxide is an inorganic compound with the chemical formula mg (oh) 2. The balanced equation is mg (oh)₂ (s) ⇌ mg²⁺ (aq) + 2oh⁻ (aq) magnesium hydroxide is sparingly soluble in water. Magnesium hydroxide is an inorganic compound which has a low solubility in water. In this video we will describe the equation mg. Magnesium Hydroxide In Water Chemical Equation.
From www.youtube.com
Equation for MgI2 + H2O (Magnesium iodide + Water) YouTube Magnesium Hydroxide In Water Chemical Equation It occurs in nature as the mineral brucite. It is also called milk of magnesia or magnesium (2+). In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. Calculate the net ionic equation for mg (oh)2 (s) + 2hcl (aq) = mgcl2 (aq) + 2h2o (l). Magnesium hydroxide can be. Magnesium Hydroxide In Water Chemical Equation.
From ar.inspiredpencil.com
Magnesium And Water Magnesium Hydroxide In Water Chemical Equation Magnesium hydroxide is an inorganic compound with the chemical formula mg (oh) 2. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. Magnesium hydroxide is an inorganic compound which has a low solubility in water. What is the balanced chemical equation that describes the dissociation of the strong base. Magnesium Hydroxide In Water Chemical Equation.
From signalticket9.pythonanywhere.com
Wonderful Magnesium Hydroxide And Nitric Acid Balanced Equation Chemical For Zinc Sulfuric Magnesium Hydroxide In Water Chemical Equation What is the balanced chemical equation that describes the dissociation of the strong base magnesium hydroxide, mg (oh)2 in. Magnesium hydroxide can be prepared using a simple chemical reaction involving magnesium chloride (mgcl₂) and sodium hydroxide (naoh). In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. Calculate the net. Magnesium Hydroxide In Water Chemical Equation.
From www.dreamstime.com
Magnesium Hydroxide Molecule 3d Rendering, Flat Molecular Structure with Chemical Formula and Magnesium Hydroxide In Water Chemical Equation In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. Thermodynamics of the reaction can be. It is a white solid. Calculate the net ionic equation for mg (oh)2 (s) + 2hcl (aq) = mgcl2 (aq) + 2h2o (l). Magnesium hydroxide is an inorganic compound which has a low solubility. Magnesium Hydroxide In Water Chemical Equation.
From www.youtube.com
Reaction between Magnesium and Water (Mg + H2O) YouTube Magnesium Hydroxide In Water Chemical Equation It is also called milk of magnesia or magnesium (2+). What is the balanced chemical equation that describes the dissociation of the strong base magnesium hydroxide, mg (oh)2 in. Magnesium hydroxide is an inorganic compound with the chemical formula mg (oh) 2. Thermodynamics of the reaction can be. It is a white solid. Magnesium hydroxide can be prepared using a. Magnesium Hydroxide In Water Chemical Equation.
From animalia-life.club
Magnesium Hydroxide Formula Magnesium Hydroxide In Water Chemical Equation It occurs in nature as the mineral brucite. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. Magnesium hydroxide can be prepared using a simple chemical reaction involving magnesium chloride (mgcl₂) and sodium hydroxide (naoh). Calculate the net ionic equation for mg (oh)2 (s) + 2hcl (aq) = mgcl2. Magnesium Hydroxide In Water Chemical Equation.
From www.chemicalslearning.com
What is the Reaction of Magnesium Chloride and Sodium Hydroxide? Magnesium Hydroxide In Water Chemical Equation Calculate the net ionic equation for mg (oh)2 (s) + 2hcl (aq) = mgcl2 (aq) + 2h2o (l). What is the balanced chemical equation that describes the dissociation of the strong base magnesium hydroxide, mg (oh)2 in. Magnesium hydroxide is an inorganic compound which has a low solubility in water. The balanced equation is mg (oh)₂ (s) ⇌ mg²⁺ (aq). Magnesium Hydroxide In Water Chemical Equation.
From www.numerade.com
SOLVED 33. Write a balanced equation for Magnesium Hydroxide in water; For your equation in Magnesium Hydroxide In Water Chemical Equation Calculate the net ionic equation for mg (oh)2 (s) + 2hcl (aq) = mgcl2 (aq) + 2h2o (l). It is a white solid. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. Thermodynamics of the reaction can be. It occurs in nature as the mineral brucite. It is also. Magnesium Hydroxide In Water Chemical Equation.
From www.youtube.com
How to Write the Formula for Magnesium hydroxide YouTube Magnesium Hydroxide In Water Chemical Equation The balanced equation is mg (oh)₂ (s) ⇌ mg²⁺ (aq) + 2oh⁻ (aq) magnesium hydroxide is sparingly soluble in water. Thermodynamics of the reaction can be. Magnesium hydroxide is an inorganic compound with the chemical formula mg (oh) 2. It is also called milk of magnesia or magnesium (2+). What is the balanced chemical equation that describes the dissociation of. Magnesium Hydroxide In Water Chemical Equation.
From www.advance-africa.com
Chemistry Notes Acid, Bases and Indicators Revision Notes & Tests Magnesium Hydroxide In Water Chemical Equation What is the balanced chemical equation that describes the dissociation of the strong base magnesium hydroxide, mg (oh)2 in. It occurs in nature as the mineral brucite. Thermodynamics of the reaction can be. The balanced equation is mg (oh)₂ (s) ⇌ mg²⁺ (aq) + 2oh⁻ (aq) magnesium hydroxide is sparingly soluble in water. In this video we will describe the. Magnesium Hydroxide In Water Chemical Equation.
From www.youtube.com
H2SO4+Mg(OH)2=H2O+MgSO4 Balanced EquationSulphuric Acid+Magnesium Hydroxide=Water+Magnesium Magnesium Hydroxide In Water Chemical Equation Calculate the net ionic equation for mg (oh)2 (s) + 2hcl (aq) = mgcl2 (aq) + 2h2o (l). Magnesium hydroxide is an inorganic compound which has a low solubility in water. What is the balanced chemical equation that describes the dissociation of the strong base magnesium hydroxide, mg (oh)2 in. In this video we will describe the equation mg (oh)2. Magnesium Hydroxide In Water Chemical Equation.
From www.numerade.com
SOLVED write a balanced chemical equation for the dissociation of solid magnesium hydroxide in Magnesium Hydroxide In Water Chemical Equation The balanced equation is mg (oh)₂ (s) ⇌ mg²⁺ (aq) + 2oh⁻ (aq) magnesium hydroxide is sparingly soluble in water. Magnesium hydroxide is an inorganic compound which has a low solubility in water. It is a white solid. Magnesium hydroxide can be prepared using a simple chemical reaction involving magnesium chloride (mgcl₂) and sodium hydroxide (naoh). Thermodynamics of the reaction. Magnesium Hydroxide In Water Chemical Equation.
From www.youtube.com
Equation for Mg(NO3)2 + H2O (Magnesium nitrate + Water) YouTube Magnesium Hydroxide In Water Chemical Equation It is a white solid. Magnesium hydroxide can be prepared using a simple chemical reaction involving magnesium chloride (mgcl₂) and sodium hydroxide (naoh). Magnesium hydroxide is an inorganic compound which has a low solubility in water. It occurs in nature as the mineral brucite. The balanced equation is mg (oh)₂ (s) ⇌ mg²⁺ (aq) + 2oh⁻ (aq) magnesium hydroxide is. Magnesium Hydroxide In Water Chemical Equation.
From signalticket9.pythonanywhere.com
Wonderful Magnesium Hydroxide And Nitric Acid Balanced Equation Chemical For Zinc Sulfuric Magnesium Hydroxide In Water Chemical Equation It occurs in nature as the mineral brucite. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. Thermodynamics of the reaction can be. Magnesium hydroxide can be prepared using a simple chemical reaction involving magnesium chloride (mgcl₂) and sodium hydroxide (naoh). It is a white solid. It is also. Magnesium Hydroxide In Water Chemical Equation.
From www.numerade.com
SOLVED Write the balanced equation for Magnesium nitride + water > magnesium hydroxide Magnesium Hydroxide In Water Chemical Equation It is also called milk of magnesia or magnesium (2+). Thermodynamics of the reaction can be. Magnesium hydroxide is an inorganic compound which has a low solubility in water. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. It is a white solid. What is the balanced chemical equation. Magnesium Hydroxide In Water Chemical Equation.
From molekula.com
Purchase Magnesium hydroxide [1309428] online • Catalog • Molekula Group Magnesium Hydroxide In Water Chemical Equation The balanced equation is mg (oh)₂ (s) ⇌ mg²⁺ (aq) + 2oh⁻ (aq) magnesium hydroxide is sparingly soluble in water. It occurs in nature as the mineral brucite. It is a white solid. Magnesium hydroxide is an inorganic compound which has a low solubility in water. Thermodynamics of the reaction can be. Calculate the net ionic equation for mg (oh)2. Magnesium Hydroxide In Water Chemical Equation.
From www.numerade.com
SOLVED Model 1 The Dissolution of Magnesium Hydroxide in Water. When solid Mg(OH)2 dissolves Magnesium Hydroxide In Water Chemical Equation Magnesium hydroxide is an inorganic compound which has a low solubility in water. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. The balanced equation is mg (oh)₂ (s) ⇌ mg²⁺ (aq) + 2oh⁻ (aq) magnesium hydroxide is sparingly soluble in water. What is the balanced chemical equation that. Magnesium Hydroxide In Water Chemical Equation.
From www.garrisonminerals.com
The Industrial Side of Magnesium Hydroxide (Part 1) Magnesium Hydroxide In Water Chemical Equation What is the balanced chemical equation that describes the dissociation of the strong base magnesium hydroxide, mg (oh)2 in. Calculate the net ionic equation for mg (oh)2 (s) + 2hcl (aq) = mgcl2 (aq) + 2h2o (l). In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. Magnesium hydroxide is. Magnesium Hydroxide In Water Chemical Equation.
From mavink.com
Magnesium And Hcl Reaction Magnesium Hydroxide In Water Chemical Equation Thermodynamics of the reaction can be. Magnesium hydroxide is an inorganic compound with the chemical formula mg (oh) 2. It is also called milk of magnesia or magnesium (2+). Magnesium hydroxide is an inorganic compound which has a low solubility in water. It is a white solid. It occurs in nature as the mineral brucite. The balanced equation is mg. Magnesium Hydroxide In Water Chemical Equation.
From chemicalforfo.blogspot.com
Chemical Formula Name Of Magnesium Hydroxide Chemical Formula Info Magnesium Hydroxide In Water Chemical Equation It occurs in nature as the mineral brucite. It is also called milk of magnesia or magnesium (2+). The balanced equation is mg (oh)₂ (s) ⇌ mg²⁺ (aq) + 2oh⁻ (aq) magnesium hydroxide is sparingly soluble in water. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. Thermodynamics of. Magnesium Hydroxide In Water Chemical Equation.
From www.chegg.com
Solved 1. Magnesium reacts with water to form magnesium Magnesium Hydroxide In Water Chemical Equation Thermodynamics of the reaction can be. It occurs in nature as the mineral brucite. What is the balanced chemical equation that describes the dissociation of the strong base magnesium hydroxide, mg (oh)2 in. Magnesium hydroxide is an inorganic compound which has a low solubility in water. Magnesium hydroxide is an inorganic compound with the chemical formula mg (oh) 2. The. Magnesium Hydroxide In Water Chemical Equation.
From www.numerade.com
SOLVED Solid magnesium hydroxide into gaseous water and solid magnesium oxide Write Magnesium Hydroxide In Water Chemical Equation Magnesium hydroxide is an inorganic compound with the chemical formula mg (oh) 2. Thermodynamics of the reaction can be. It occurs in nature as the mineral brucite. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. It is a white solid. The balanced equation is mg (oh)₂ (s) ⇌. Magnesium Hydroxide In Water Chemical Equation.
From brainly.in
9.Write the chemical formula (i) Hydrochloric acid (ii) Magnesium hydroxide (iii) Aluminum Magnesium Hydroxide In Water Chemical Equation It occurs in nature as the mineral brucite. The balanced equation is mg (oh)₂ (s) ⇌ mg²⁺ (aq) + 2oh⁻ (aq) magnesium hydroxide is sparingly soluble in water. It is a white solid. Calculate the net ionic equation for mg (oh)2 (s) + 2hcl (aq) = mgcl2 (aq) + 2h2o (l). Magnesium hydroxide is an inorganic compound which has a. Magnesium Hydroxide In Water Chemical Equation.
From www.youtube.com
How to write the formula for magnesium hydroxide YouTube Magnesium Hydroxide In Water Chemical Equation Magnesium hydroxide is an inorganic compound with the chemical formula mg (oh) 2. It occurs in nature as the mineral brucite. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. What is the balanced chemical equation that describes the dissociation of the strong base magnesium hydroxide, mg (oh)2 in.. Magnesium Hydroxide In Water Chemical Equation.
From www.numerade.com
SOLVEDFor the dissolution of magnesium hydroxide in water (a) Write a chemical equation for Magnesium Hydroxide In Water Chemical Equation It occurs in nature as the mineral brucite. Thermodynamics of the reaction can be. Magnesium hydroxide is an inorganic compound which has a low solubility in water. Calculate the net ionic equation for mg (oh)2 (s) + 2hcl (aq) = mgcl2 (aq) + 2h2o (l). The balanced equation is mg (oh)₂ (s) ⇌ mg²⁺ (aq) + 2oh⁻ (aq) magnesium hydroxide. Magnesium Hydroxide In Water Chemical Equation.
From www.researchgate.net
Schematic diagram of the reactions on the surface of the magnesium... Download Scientific Diagram Magnesium Hydroxide In Water Chemical Equation In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. Magnesium hydroxide is an inorganic compound with the chemical formula mg (oh) 2. It occurs in nature as the mineral brucite. The balanced equation is mg (oh)₂ (s) ⇌ mg²⁺ (aq) + 2oh⁻ (aq) magnesium hydroxide is sparingly soluble in. Magnesium Hydroxide In Water Chemical Equation.
From solvedlib.com
Magnesium hydroxide reacts with hydrochloric acid to … SolvedLib Magnesium Hydroxide In Water Chemical Equation Magnesium hydroxide is an inorganic compound which has a low solubility in water. Magnesium hydroxide can be prepared using a simple chemical reaction involving magnesium chloride (mgcl₂) and sodium hydroxide (naoh). It occurs in nature as the mineral brucite. It is also called milk of magnesia or magnesium (2+). In this video we will describe the equation mg (oh)2 +. Magnesium Hydroxide In Water Chemical Equation.
From www.thesciencehive.co.uk
Mass and Mole Calculations (AQA) — the science sauce Magnesium Hydroxide In Water Chemical Equation Magnesium hydroxide is an inorganic compound with the chemical formula mg (oh) 2. In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. Thermodynamics of the reaction can be. Calculate the net ionic equation for mg (oh)2 (s) + 2hcl (aq) = mgcl2 (aq) + 2h2o (l). The balanced equation. Magnesium Hydroxide In Water Chemical Equation.
From testbook.com
Magnesium Hydroxide Definition, Symbol, Examples, Structure Magnesium Hydroxide In Water Chemical Equation Magnesium hydroxide is an inorganic compound with the chemical formula mg (oh) 2. It occurs in nature as the mineral brucite. Thermodynamics of the reaction can be. Calculate the net ionic equation for mg (oh)2 (s) + 2hcl (aq) = mgcl2 (aq) + 2h2o (l). It is also called milk of magnesia or magnesium (2+). In this video we will. Magnesium Hydroxide In Water Chemical Equation.
From www.numerade.com
SOLVED Question 5 What is the equilibrium constant expression for the dissolution of solid Magnesium Hydroxide In Water Chemical Equation It is a white solid. Magnesium hydroxide can be prepared using a simple chemical reaction involving magnesium chloride (mgcl₂) and sodium hydroxide (naoh). Calculate the net ionic equation for mg (oh)2 (s) + 2hcl (aq) = mgcl2 (aq) + 2h2o (l). It occurs in nature as the mineral brucite. Thermodynamics of the reaction can be. Magnesium hydroxide is an inorganic. Magnesium Hydroxide In Water Chemical Equation.
From infinitylearn.com
Magnesium hydroxide Formula Infinity Learn Magnesium Hydroxide In Water Chemical Equation In this video we will describe the equation mg (oh)2 + h2o and write what happens when mg (oh)2 is. The balanced equation is mg (oh)₂ (s) ⇌ mg²⁺ (aq) + 2oh⁻ (aq) magnesium hydroxide is sparingly soluble in water. It is a white solid. It occurs in nature as the mineral brucite. It is also called milk of magnesia. Magnesium Hydroxide In Water Chemical Equation.
From www.youtube.com
Equation for Magnesium Hydroxide Dissolving in Water Mg(OH)2 + H2O YouTube Magnesium Hydroxide In Water Chemical Equation Magnesium hydroxide is an inorganic compound which has a low solubility in water. Calculate the net ionic equation for mg (oh)2 (s) + 2hcl (aq) = mgcl2 (aq) + 2h2o (l). Thermodynamics of the reaction can be. Magnesium hydroxide can be prepared using a simple chemical reaction involving magnesium chloride (mgcl₂) and sodium hydroxide (naoh). It occurs in nature as. Magnesium Hydroxide In Water Chemical Equation.
From www.youtube.com
How to Balance Mg + H2O = MgO + H2 Magnesium + Water (steam) YouTube Magnesium Hydroxide In Water Chemical Equation It is a white solid. Magnesium hydroxide is an inorganic compound with the chemical formula mg (oh) 2. It is also called milk of magnesia or magnesium (2+). It occurs in nature as the mineral brucite. Magnesium hydroxide can be prepared using a simple chemical reaction involving magnesium chloride (mgcl₂) and sodium hydroxide (naoh). What is the balanced chemical equation. Magnesium Hydroxide In Water Chemical Equation.