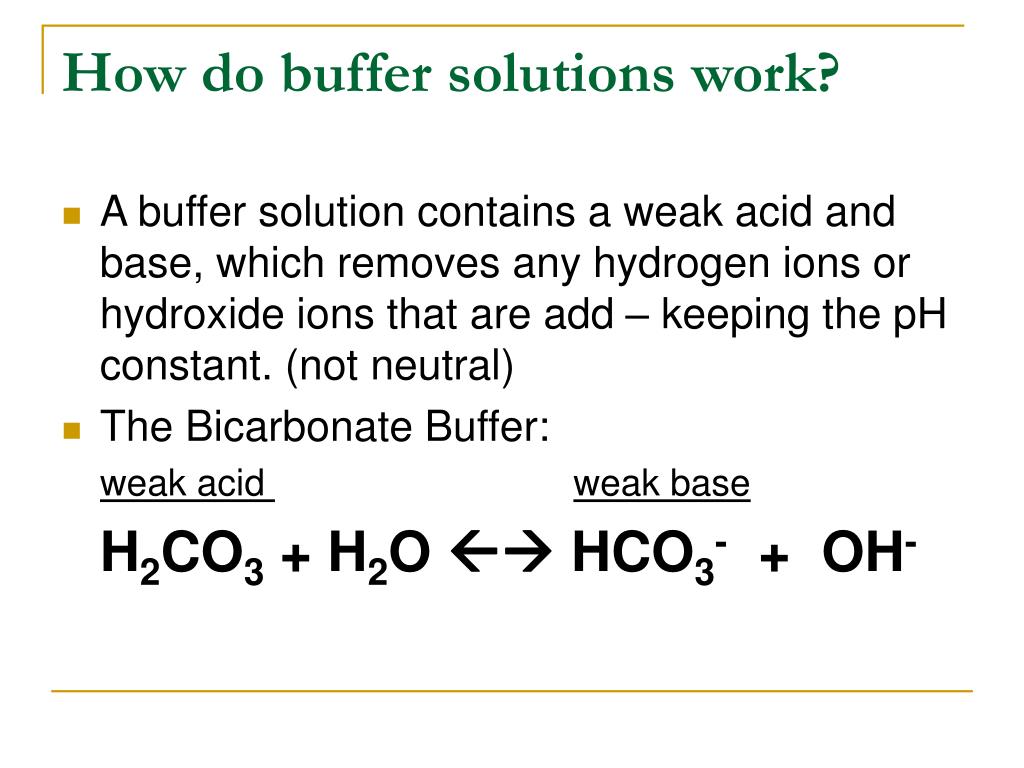
What Is A Buffer Purpose . It is able to neutralize small. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer solution resists a change in ph from the addition of a small amount of acid or base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that resists sudden changes in ph. A buffer is a solution that maintains the. It is able to neutralize small. The essential component of a buffer system is a. Buffers do so by being composed of certain pairs of solutes:. Buffer definition and examples in chemistry.
from www.slideserve.com
Buffers do so by being composed of certain pairs of solutes:. It is able to neutralize small. It is able to neutralize small. A buffer solution resists a change in ph from the addition of a small amount of acid or base. The essential component of a buffer system is a. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer definition and examples in chemistry. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that resists sudden changes in ph.
PPT Buffers PowerPoint Presentation, free download ID6787517
What Is A Buffer Purpose The essential component of a buffer system is a. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the. A buffer is a solution that resists sudden changes in ph. A buffer solution resists a change in ph from the addition of a small amount of acid or base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. It is able to neutralize small. Buffer definition and examples in chemistry. Buffers do so by being composed of certain pairs of solutes:. The essential component of a buffer system is a.
From chem.libretexts.org
14.6 Buffers Chemistry LibreTexts What Is A Buffer Purpose Buffer definition and examples in chemistry. A buffer solution resists a change in ph from the addition of a small amount of acid or base. It is able to neutralize small. A buffer is a solution that maintains the. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The mechanism. What Is A Buffer Purpose.
From www.slideserve.com
PPT All About Buffers PowerPoint Presentation, free download ID1321295 What Is A Buffer Purpose Buffer definition and examples in chemistry. It is able to neutralize small. The essential component of a buffer system is a. A buffer is a solution that resists sudden changes in ph. A buffer is a solution that maintains the. A buffer solution resists a change in ph from the addition of a small amount of acid or base. The. What Is A Buffer Purpose.
From mydigitalkemistry.com
What is a buffer solution simple definition? Free Chemistry Learning What Is A Buffer Purpose Buffers do so by being composed of certain pairs of solutes:. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that resists sudden changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components.. What Is A Buffer Purpose.
From www.slideshare.net
Buffer system What Is A Buffer Purpose A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the. Buffer definition and examples in chemistry. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that resists sudden. What Is A Buffer Purpose.
From techjury.net
What Is Buffering? How and Why Does it Happen? What Is A Buffer Purpose It is able to neutralize small. A buffer is a solution that resists sudden changes in ph. Buffers do so by being composed of certain pairs of solutes:. The essential component of a buffer system is a. It is able to neutralize small. A buffer solution resists a change in ph from the addition of a small amount of acid. What Is A Buffer Purpose.
From ar.inspiredpencil.com
How To Use A Nail Buffer What Is A Buffer Purpose Buffer definition and examples in chemistry. A buffer is a solution that resists sudden changes in ph. The essential component of a buffer system is a. It is able to neutralize small. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer solution resists a change in ph from the addition of a small amount. What Is A Buffer Purpose.
From www.youtube.com
BUFFER SOLUTIONS HOW TO MAKE BUFFER ? TYPES OF BUFFER HOW BUFFERS What Is A Buffer Purpose It is able to neutralize small. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution that resists sudden changes in ph. Buffers do so by being composed of certain pairs of solutes:. It is able to neutralize small. Buffer definition and examples in chemistry. A buffer is a solution that can. What Is A Buffer Purpose.
From www.acunetix.com
What Is a Buffer Overflow What Is A Buffer Purpose It is able to neutralize small. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffer definition and examples in chemistry. A buffer is a solution that maintains the. It is able to neutralize small. A buffer is a solution that can resist ph change upon the addition of an. What Is A Buffer Purpose.
From www.slideserve.com
PPT Fluid, Electrolyte, and AcidBase Balance PowerPoint Presentation What Is A Buffer Purpose It is able to neutralize small. It is able to neutralize small. A buffer is a solution that maintains the. A buffer is a solution that resists sudden changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer definition and examples in chemistry. The essential component of. What Is A Buffer Purpose.
From www.chegg.com
Solved Working with Buffers Purpose The purpose of this What Is A Buffer Purpose Buffer definition and examples in chemistry. A buffer is a solution that maintains the. Buffers do so by being composed of certain pairs of solutes:. The mechanism involves a buffer, a solution that resists dramatic changes in ph. The essential component of a buffer system is a. A buffer is a solution that can resist ph change upon the addition. What Is A Buffer Purpose.
From kyvokedymolyb.modellervefiyatlar.com
Acid Base Buffers What Is A Buffer Purpose A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that maintains the. Buffers do so by being composed of certain pairs of solutes:. The mechanism involves. What Is A Buffer Purpose.
From loexxrihd.blob.core.windows.net
What Is The Function Of A Buffer What Is A Buffer Made From at What Is A Buffer Purpose A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution resists a change in ph from the addition of a small amount of acid or base. It is able to neutralize small. The essential component of a buffer system is a. Buffer definition and examples in chemistry. A. What Is A Buffer Purpose.
From webmis.highland.cc.il.us
Buffers What Is A Buffer Purpose A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The mechanism involves a buffer, a solution that resists dramatic changes in ph. It is able to neutralize small. A buffer is a solution that maintains the. A buffer solution resists a change in ph from the addition of a small. What Is A Buffer Purpose.
From www.slideshare.net
Buffers in chemical analysis, types of buffers What Is A Buffer Purpose The essential component of a buffer system is a. It is able to neutralize small. Buffers do so by being composed of certain pairs of solutes:. A buffer is a solution that maintains the. Buffer definition and examples in chemistry. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution that can. What Is A Buffer Purpose.
From ridtpoof-lano.blogspot.com
What Is The Most Powerful Buffer System In The Body Maria Ma Coiffure What Is A Buffer Purpose Buffers do so by being composed of certain pairs of solutes:. A buffer is a solution that resists sudden changes in ph. It is able to neutralize small. The essential component of a buffer system is a. A buffer solution resists a change in ph from the addition of a small amount of acid or base. It is able to. What Is A Buffer Purpose.
From www.youtube.com
What is Buffer ? Why Buffer and TriState Buffers are used in Digital What Is A Buffer Purpose The essential component of a buffer system is a. A buffer is a solution that resists sudden changes in ph. The mechanism involves a buffer, a solution that resists dramatic changes in ph. It is able to neutralize small. Buffer definition and examples in chemistry. A buffer solution resists a change in ph from the addition of a small amount. What Is A Buffer Purpose.
From www.youtube.com
Buffer action in the blood YouTube What Is A Buffer Purpose A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution resists a change in ph from the addition of a small amount of acid or base. The mechanism involves a buffer, a solution that resists dramatic changes in ph. It is able to neutralize small. A buffer is. What Is A Buffer Purpose.
From www.slideserve.com
PPT Chapter 26 Balance PowerPoint Presentation, free download ID What Is A Buffer Purpose Buffer definition and examples in chemistry. A buffer is a solution that maintains the. The essential component of a buffer system is a. A buffer is a solution that resists sudden changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The mechanism involves a buffer, a solution. What Is A Buffer Purpose.
From psiberg.com
Buffer Solutions Principle and Mechanism of their Action PSIBERG What Is A Buffer Purpose A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. A buffer is a solution that resists sudden changes in ph. A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffer definition and examples in. What Is A Buffer Purpose.
From www.slideserve.com
PPT Buffers PowerPoint Presentation, free download ID6787517 What Is A Buffer Purpose A buffer solution resists a change in ph from the addition of a small amount of acid or base. Buffers do so by being composed of certain pairs of solutes:. Buffer definition and examples in chemistry. The mechanism involves a buffer, a solution that resists dramatic changes in ph. A buffer is a solution that maintains the. The essential component. What Is A Buffer Purpose.
From www.reagents.com
Buffers General Purpose Buffers Reagents What Is A Buffer Purpose It is able to neutralize small. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers do so by being composed of certain pairs of solutes:. A buffer is a solution. What Is A Buffer Purpose.
From www.slideserve.com
PPT Buffer Solutions PowerPoint Presentation, free download ID5799007 What Is A Buffer Purpose A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers do so by being composed of certain pairs of solutes:. The mechanism involves a buffer, a solution that resists dramatic changes in ph. It is able to neutralize small. A buffer is a solution that can resist ph change upon. What Is A Buffer Purpose.
From www.slideserve.com
PPT Stock PowerPoint Presentation, free download ID1663896 What Is A Buffer Purpose A buffer is a solution that maintains the. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer definition and examples in chemistry. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. Buffers do. What Is A Buffer Purpose.
From design.udlvirtual.edu.pe
How Does A Circular Buffer Work Design Talk What Is A Buffer Purpose A buffer solution resists a change in ph from the addition of a small amount of acid or base. The essential component of a buffer system is a. Buffers do so by being composed of certain pairs of solutes:. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer. What Is A Buffer Purpose.
From sciencenotes.org
Buffer Definition and Examples in Chemistry What Is A Buffer Purpose Buffer definition and examples in chemistry. It is able to neutralize small. The essential component of a buffer system is a. A buffer is a solution that maintains the. A buffer solution resists a change in ph from the addition of a small amount of acid or base. The mechanism involves a buffer, a solution that resists dramatic changes in. What Is A Buffer Purpose.
From www.slideserve.com
PPT In the name of god PowerPoint Presentation, free download ID What Is A Buffer Purpose Buffers do so by being composed of certain pairs of solutes:. A buffer solution resists a change in ph from the addition of a small amount of acid or base. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The mechanism involves a buffer, a solution that resists dramatic changes. What Is A Buffer Purpose.
From loexxrihd.blob.core.windows.net
What Is The Function Of A Buffer What Is A Buffer Made From at What Is A Buffer Purpose The essential component of a buffer system is a. A buffer is a solution that maintains the. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers do so by being composed of certain pairs of solutes:. It is able to neutralize small. It is able to neutralize small. The. What Is A Buffer Purpose.
From scienceinfo.com
Buffer Solution Types, Properties, and Uses What Is A Buffer Purpose The essential component of a buffer system is a. A buffer is a solution that resists sudden changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffer definition and examples in chemistry. A buffer is a solution that maintains the. A buffer solution resists a change in. What Is A Buffer Purpose.
From loexxrihd.blob.core.windows.net
What Is The Function Of A Buffer What Is A Buffer Made From at What Is A Buffer Purpose It is able to neutralize small. Buffer definition and examples in chemistry. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. Buffers do so by being composed of certain pairs of solutes:. The essential component of a buffer system is a. A buffer solution resists a change in ph from. What Is A Buffer Purpose.
From slidetodoc.com
Introduction to Buffers These solutions contain relatively high What Is A Buffer Purpose A buffer is a solution that maintains the. The essential component of a buffer system is a. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution resists a. What Is A Buffer Purpose.
From www.youtube.com
Tristate buffer what is tristate buffer inverting non inverting What Is A Buffer Purpose A buffer solution resists a change in ph from the addition of a small amount of acid or base. A buffer is a solution that resists sudden changes in ph. A buffer is a solution that maintains the. The essential component of a buffer system is a. It is able to neutralize small. Buffers do so by being composed of. What Is A Buffer Purpose.
From www.youtube.com
Introduction to Buffer System Regulation of pH Acid Base Balance What Is A Buffer Purpose A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer is a solution that resists sudden changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. It is able to neutralize small. Buffers do so by being. What Is A Buffer Purpose.
From bitesizebio.com
Laemmli Buffer What Is It for Anyway? Bitesize Bio What Is A Buffer Purpose A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. A buffer solution resists a change in ph from the addition of a small amount of acid or base. A buffer is a solution that resists sudden changes in ph. Buffers do so by being composed of certain pairs of solutes:.. What Is A Buffer Purpose.
From www.youtube.com
18.2.1 Describe the composition of a buffer solution and explain its What Is A Buffer Purpose A buffer is a solution that maintains the. A buffer is a solution that resists sudden changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The mechanism involves a buffer, a solution that resists dramatic changes in ph. It is able to neutralize small. Buffers do so. What Is A Buffer Purpose.
From woodlandinfo.org
Riparian Buffers Agroforestry for Any Property UWMadison Extension What Is A Buffer Purpose A buffer solution resists a change in ph from the addition of a small amount of acid or base. A buffer is a solution that maintains the. A buffer is a solution that resists sudden changes in ph. A buffer is a solution that can resist ph change upon the addition of an acidic or basic components. The essential component. What Is A Buffer Purpose.