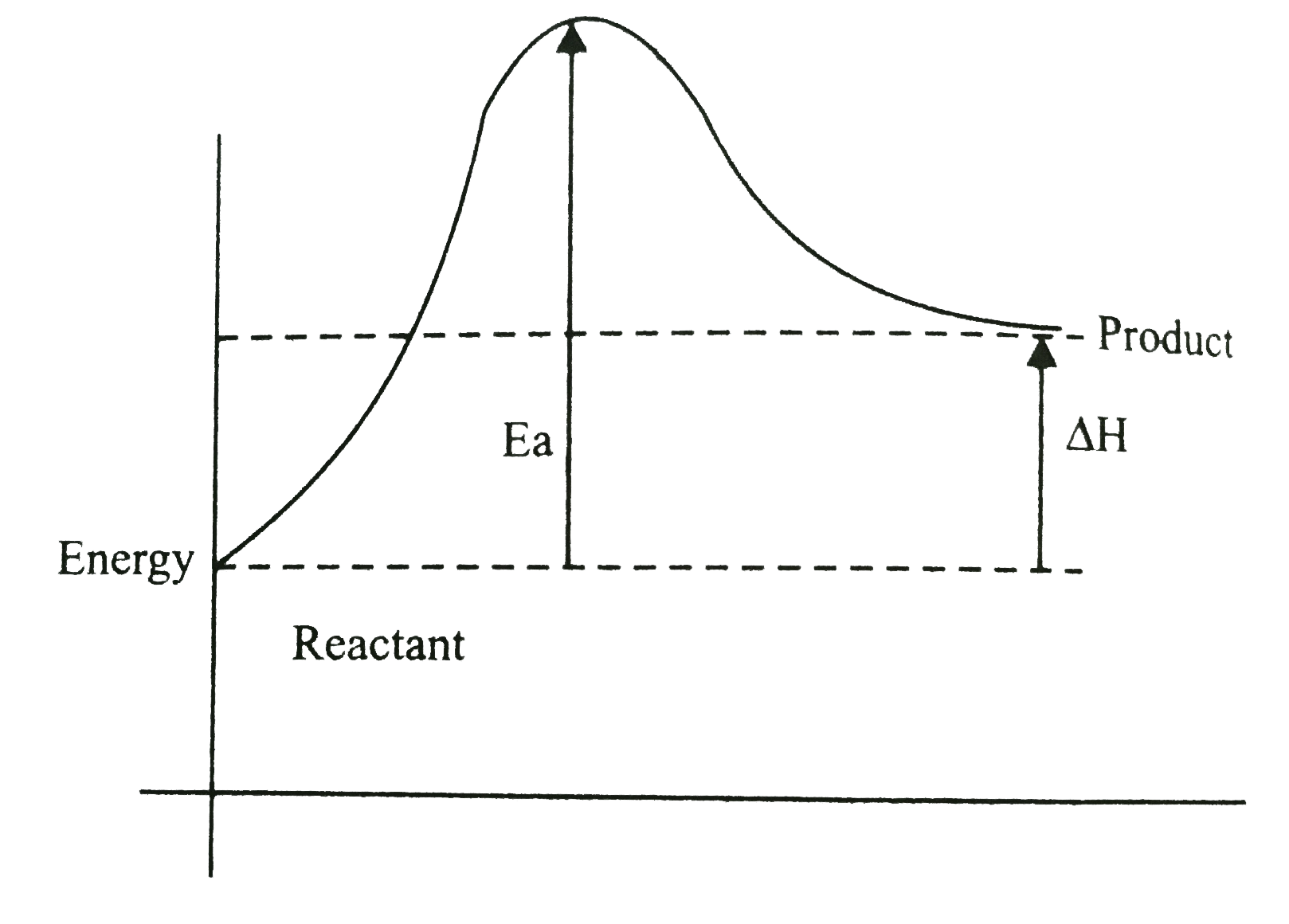
Endothermic Reaction Line . Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. In an energy profile diagram, an endothermic reaction is represented by a diagram that shows the initial energy level of the reactants being lower than the final energy level of the products. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. This is because energy is taken in from the surroundings. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. \text{mol}\) of calcium oxide and \(1 \:. The heat absorbed by the reaction provides the activation energy needed for the reaction to occur. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Because heat is absorbed, endothermic reactions feel cold. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. Describe three ways to speed up a reaction. \text{mol}\) of calcium carbonate decomposes into \(1 \: An endothermic reaction is a type of chemical reaction that absorbs heat from its surroundings, resulting in a decrease in temperature. The energy level increases in an endothermic reaction.
from www.doubtnut.com
In an energy profile diagram, an endothermic reaction is represented by a diagram that shows the initial energy level of the reactants being lower than the final energy level of the products. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. Describe three ways to speed up a reaction. The energy level increases in an endothermic reaction. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Because heat is absorbed, endothermic reactions feel cold. An endothermic reaction is a type of chemical reaction that absorbs heat from its surroundings, resulting in a decrease in temperature. The heat absorbed by the reaction provides the activation energy needed for the reaction to occur.
For an endothermic reaction, where Delta H represents the enthalpy of
Endothermic Reaction Line These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. \text{mol}\) of calcium carbonate decomposes into \(1 \: An endothermic reaction is a type of chemical reaction that absorbs heat from its surroundings, resulting in a decrease in temperature. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. This is because energy is taken in from the surroundings. The energy level increases in an endothermic reaction. In an energy profile diagram, an endothermic reaction is represented by a diagram that shows the initial energy level of the reactants being lower than the final energy level of the products. Because heat is absorbed, endothermic reactions feel cold. The heat absorbed by the reaction provides the activation energy needed for the reaction to occur. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. Describe three ways to speed up a reaction. \text{mol}\) of calcium oxide and \(1 \:.
From eduinput.com
Endothermic ReactionsCharacteristics, Identification, and Examples Endothermic Reaction Line The heat absorbed by the reaction provides the activation energy needed for the reaction to occur. In an energy profile diagram, an endothermic reaction is represented by a diagram that shows the initial energy level of the reactants being lower than the final energy level of the products. Describe three ways to speed up a reaction. Revise and understand what. Endothermic Reaction Line.
From www.vecteezy.com
endothermic reaction line icon with test tube 8235749 Vector Art at Endothermic Reaction Line Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. The energy level increases in an endothermic reaction. \text{mol}\) of calcium oxide and \(1 \:. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The changes in energy that occur during a chemical reaction can be seen. Endothermic Reaction Line.
From www.doubtnut.com
For an endothermic reaction, where Delta H represents the enthalpy of Endothermic Reaction Line \text{mol}\) of calcium carbonate decomposes into \(1 \: Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. The heat absorbed by the reaction provides the activation energy needed for the reaction to occur. Because heat is absorbed, endothermic reactions feel cold. Describe three ways to speed up a reaction. In an. Endothermic Reaction Line.
From www.slideserve.com
PPT Phase Changes PowerPoint Presentation, free download ID2202556 Endothermic Reaction Line Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. An endothermic reaction is a type of chemical reaction that absorbs heat from its surroundings, resulting in a decrease in temperature. The heat absorbed by the reaction provides the activation energy needed for the reaction to occur. The energy level increases in. Endothermic Reaction Line.
From www.shutterstock.com
124 Endotherm Reaction Images, Stock Photos & Vectors Shutterstock Endothermic Reaction Line An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. The heat absorbed by the reaction provides the activation energy needed for the reaction to occur. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to. Endothermic Reaction Line.
From elecdiags.com
The Significance and Use of Endothermic Reaction Profile Diagrams in Endothermic Reaction Line \text{mol}\) of calcium oxide and \(1 \:. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. Describe three ways to speed up a reaction. In an energy profile diagram, an. Endothermic Reaction Line.
From www.doubtnut.com
For an endothermic reaction energy of activation is E(a) and enthlpy o Endothermic Reaction Line Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. \text{mol}\) of calcium carbonate decomposes into \(1 \: \text{mol}\) of calcium oxide and \(1 \:. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. The energy level increases. Endothermic Reaction Line.
From techschematic.com
The Enthalpy Diagram of an Endothermic Reaction Explained Endothermic Reaction Line Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. An endothermic reaction is a type of chemical reaction that absorbs heat from its surroundings, resulting in a decrease in temperature. In an energy profile diagram, an endothermic reaction is represented by a diagram that shows the initial energy level of the. Endothermic Reaction Line.
From testbook.com
Endothermic Reaction Learn Definition, Reagents, Formula here Endothermic Reaction Line Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. Because heat is absorbed, endothermic reactions feel cold. \text{mol}\) of. Endothermic Reaction Line.
From www.alamy.com
endothermic reaction line icon with test tubes Stock Vector Image & Art Endothermic Reaction Line These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. This is because energy is taken in from the surroundings. Revise and. Endothermic Reaction Line.
From resolutionsforyou.com
The Journey of an Endothermic Reaction Understanding the Reaction Endothermic Reaction Line In an energy profile diagram, an endothermic reaction is represented by a diagram that shows the initial energy level of the reactants being lower than the final energy level of the products. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and. Endothermic Reaction Line.
From mareeromana.blogspot.com
12+ Endothermic Enthalpy Diagram MareeRomana Endothermic Reaction Line Because heat is absorbed, endothermic reactions feel cold. An endothermic reaction is a type of chemical reaction that absorbs heat from its surroundings, resulting in a decrease in temperature. In an energy profile diagram, an endothermic reaction is represented by a diagram that shows the initial energy level of the reactants being lower than the final energy level of the. Endothermic Reaction Line.
From www.slideserve.com
PPT Chapter 12 “Heat in Chemical Reactions” PowerPoint Presentation Endothermic Reaction Line In an energy profile diagram, an endothermic reaction is represented by a diagram that shows the initial energy level of the reactants being lower than the final energy level of the products. The heat absorbed by the reaction provides the activation energy needed for the reaction to occur. Revise and understand what endothermic and exothermic reactions are and how the. Endothermic Reaction Line.
From www.doubtnut.com
An endothermic reaction with high activation energy for the forward re Endothermic Reaction Line This is because energy is taken in from the surroundings. An endothermic reaction is a type of chemical reaction that absorbs heat from its surroundings, resulting in a decrease in temperature. \text{mol}\) of calcium oxide and \(1 \:. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy,. Endothermic Reaction Line.
From slideplayer.com
Endothermic Vs. Exothermic Reaction Graphs ppt download Endothermic Reaction Line The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. The energy level increases in an endothermic reaction. An endothermic reaction is a type of chemical reaction that absorbs heat from its surroundings, resulting in a decrease in temperature. The heat absorbed by the reaction provides the activation energy needed. Endothermic Reaction Line.
From revisechemistry.uk
Exothermic and Endothermic Reactions AQA C5 revisechemistry.uk Endothermic Reaction Line These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. In an energy profile diagram, an endothermic reaction is represented by a diagram that shows the initial energy level of the reactants being lower than the. Endothermic Reaction Line.
From www.expii.com
Energy Diagram — Overview & Parts Expii Endothermic Reaction Line The energy level increases in an endothermic reaction. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. This is because energy is taken in from the surroundings. The heat absorbed by the reaction provides the activation energy needed for the reaction to occur. In an energy profile diagram, an endothermic reaction is represented by a. Endothermic Reaction Line.
From mmerevise.co.uk
Enthalpy Changes and Calorimetry MME Endothermic Reaction Line \text{mol}\) of calcium oxide and \(1 \:. The heat absorbed by the reaction provides the activation energy needed for the reaction to occur. Because heat is absorbed, endothermic reactions feel cold. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Describe three ways to speed up a reaction. This is because energy is taken in. Endothermic Reaction Line.
From www.doubtnut.com
An endothermic reaction with high activation energy for the forward re Endothermic Reaction Line In an energy profile diagram, an endothermic reaction is represented by a diagram that shows the initial energy level of the reactants being lower than the final energy level of the products. The energy level increases in an endothermic reaction. \text{mol}\) of calcium oxide and \(1 \:. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs. Endothermic Reaction Line.
From www.slideserve.com
PPT Chemical Reactions PowerPoint Presentation, free download ID431456 Endothermic Reaction Line The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. \text{mol}\) of calcium carbonate decomposes into \(1 \: Describe three ways to speed up a reaction. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. An endothermic reaction. Endothermic Reaction Line.
From www.youtube.com
Endothermic Reactions YouTube Endothermic Reaction Line Describe three ways to speed up a reaction. \text{mol}\) of calcium oxide and \(1 \:. Because heat is absorbed, endothermic reactions feel cold. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. The energy level increases in an endothermic reaction. An endothermic reaction is a type of chemical reaction that absorbs heat from its surroundings,. Endothermic Reaction Line.
From www.shutterstock.com
diagramme de réaction endothermique de l'énergie image vectorielle de Endothermic Reaction Line Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. Because heat is absorbed, endothermic reactions feel. Endothermic Reaction Line.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Reaction Line \text{mol}\) of calcium carbonate decomposes into \(1 \: The heat absorbed by the reaction provides the activation energy needed for the reaction to occur. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. Because heat is absorbed, endothermic reactions feel cold. The changes in energy that occur during a chemical reaction can be seen by. Endothermic Reaction Line.
From wiringdatabaseinfo.blogspot.com
Energy Diagram For Endothermic Reaction Wiring Site Resource Endothermic Reaction Line Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. This is because energy is taken in from the surroundings. The energy level increases in an endothermic reaction. In an energy profile diagram, an endothermic reaction is represented. Endothermic Reaction Line.
From www.studyorgo.com
How to Interpret Thermodynamics of Reactions Endothermic Reaction Line Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. This is because energy is taken in from the surroundings. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph.. Endothermic Reaction Line.
From www.pinterest.co.uk
Endothermic and Exothermic Reactions Lab ⋆ Exothermic Endothermic Reaction Line The energy level increases in an endothermic reaction. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Describe three ways to speed up a reaction. The heat absorbed by the reaction provides the activation energy needed for the reaction to occur. In an energy profile diagram, an endothermic reaction. Endothermic Reaction Line.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Line These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. An endothermic reaction is a type of chemical reaction that absorbs heat from its surroundings, resulting in a decrease in temperature. The heat absorbed by the reaction provides. Endothermic Reaction Line.
From manualpartsynaxis123.z13.web.core.windows.net
Exothermic And Endothermic Energy Diagrams Endothermic Reaction Line The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Because heat is absorbed, endothermic reactions feel cold. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include. Endothermic Reaction Line.
From vhmsscience.weebly.com
Endo/Exothermic Reactions VISTA HEIGHTS 8TH GRADE SCIENCE Endothermic Reaction Line The heat absorbed by the reaction provides the activation energy needed for the reaction to occur. The energy level increases in an endothermic reaction. This is because energy is taken in from the surroundings. In an energy profile diagram, an endothermic reaction is represented by a diagram that shows the initial energy level of the reactants being lower than the. Endothermic Reaction Line.
From www.thoughtco.com
Endothermic and Exothermic Chemical Reactions Endothermic Reaction Line Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. Endothermic reactions are chemical reactions in which the reactants absorb heat energy from the surroundings to form products. These reactions lower the temperature of their surrounding area, thereby. Endothermic Reaction Line.
From sciencenotes.org
Endothermic Reactions Definition and Examples Endothermic Reaction Line An endothermic reaction is a type of chemical reaction that absorbs heat from its surroundings, resulting in a decrease in temperature. The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. In an energy profile diagram, an endothermic reaction is represented by a diagram that shows the initial energy level. Endothermic Reaction Line.
From www.chemistrylearner.com
Endothermic Reaction Definition, Equation, Graph & Examples Endothermic Reaction Line These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. Describe three ways to speed up a reaction. An endothermic reaction is a chemical reaction that absorbs thermal energy from its surroundings. The energy level increases in an endothermic reaction. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy. Endothermic Reaction Line.
From www.chegg.com
Solved The figure below illustrates the difference between Endothermic Reaction Line \text{mol}\) of calcium carbonate decomposes into \(1 \: The energy level increases in an endothermic reaction. This is because energy is taken in from the surroundings. Differentiate between reversible exothermic, irreversible exothermic, and endothermic reactions and draw appropriate graphs to represent each which include axis labels, activation energy, and the effect a catalyst would have on the graph. These reactions. Endothermic Reaction Line.
From www.worksheetsplanet.com
What is an Endothermic Reaction Definition & Example Endothermic Reaction Line Describe three ways to speed up a reaction. The energy level increases in an endothermic reaction. These reactions lower the temperature of their surrounding area, thereby creating a cooling effect. The heat absorbed by the reaction provides the activation energy needed for the reaction to occur. \text{mol}\) of calcium carbonate decomposes into \(1 \: \text{mol}\) of calcium oxide and \(1. Endothermic Reaction Line.
From keystagewiki.com
Endothermic Key Stage Wiki Endothermic Reaction Line The changes in energy that occur during a chemical reaction can be seen by examining the changes in chemical bonding. Describe three ways to speed up a reaction. Revise and understand what endothermic and exothermic reactions are and how the two reactions affect energy transfer to or from their. Because heat is absorbed, endothermic reactions feel cold. The heat absorbed. Endothermic Reaction Line.