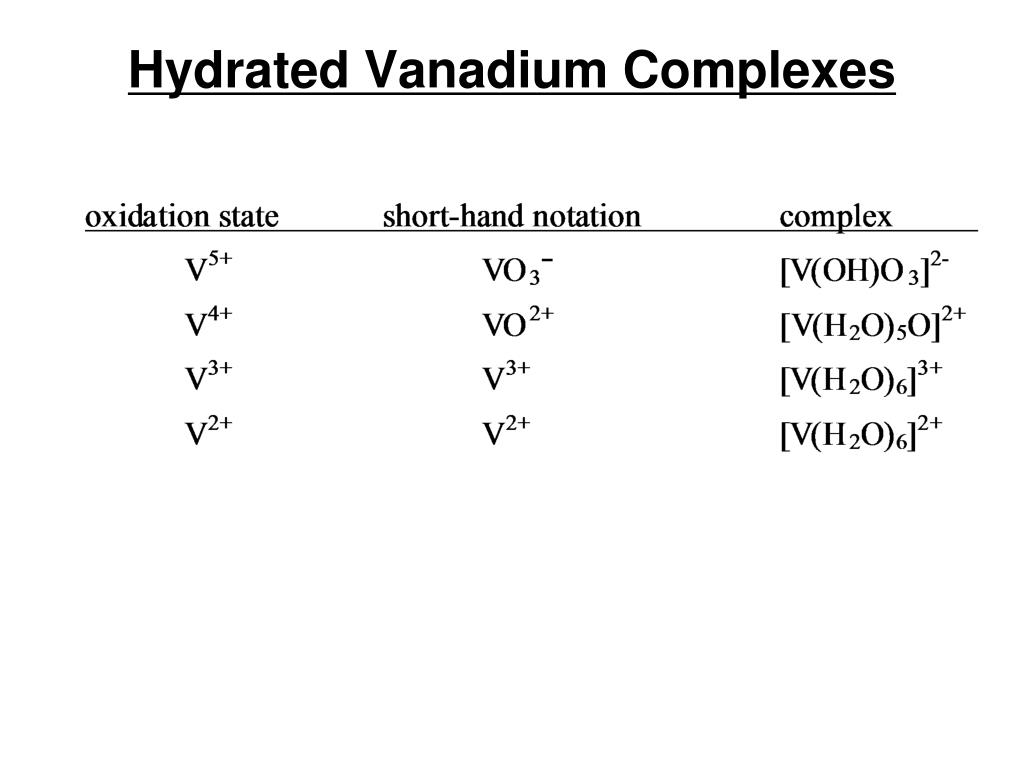
Standard Reduction Potential Vanadium . It starts with a bit of description, and. This section looks at ways of changing between them. All other species are aqueous. In this lab, v5+ is reduced to v4+, v3+, v2+. Based on your results and the standard reduction potential of each addition, you should be able to. The different oxidation states of vanadium are evident due to color changes, and can be separated by. Vanadium is reduced from an oxidation number of +3 to +2 in half equation 3. Vanadium has oxidation states in its compounds of +5, +4, +3 and +2. Values are from the following sources: The e θ value for half equation 2 is more negative than the e θ for half equation 3. Reduction reactions in acidic solution are written using h + in place of. The following table provides e o for selected reduction reactions. The values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for the following. Solids, gases, and liquids are identified; 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:.
from www.slideserve.com
In this lab, v5+ is reduced to v4+, v3+, v2+. Zn is the best reducing agent. The values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for the following. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The e θ value for half equation 2 is more negative than the e θ for half equation 3. The following table provides e o for selected reduction reactions. Based on your results and the standard reduction potential of each addition, you should be able to. It starts with a bit of description, and. Values are from the following sources: The different oxidation states of vanadium are evident due to color changes, and can be separated by.
PPT The Oxidation States of Vanadium PowerPoint Presentation, free
Standard Reduction Potential Vanadium Solids, gases, and liquids are identified; Reduction reactions in acidic solution are written using h + in place of. The values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for the following. Zn is the best reducing agent. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The different oxidation states of vanadium are evident due to color changes, and can be separated by. Solids, gases, and liquids are identified; All other species are aqueous. In this lab, v5+ is reduced to v4+, v3+, v2+. In this lab, we will be employing zinc, tin, and silver metals to reduce vanadium. The following table provides e o for selected reduction reactions. Values are from the following sources: It starts with a bit of description, and. Vanadium is reduced from an oxidation number of +3 to +2 in half equation 3. Based on your results and the standard reduction potential of each addition, you should be able to. Vanadium has oxidation states in its compounds of +5, +4, +3 and +2.
From www.studypool.com
SOLUTION Table of standard reduction potentials 1 Studypool Standard Reduction Potential Vanadium The following table provides e o for selected reduction reactions. The different oxidation states of vanadium are evident due to color changes, and can be separated by. Based on your results and the standard reduction potential of each addition, you should be able to. Reduction reactions in acidic solution are written using h + in place of. Vanadium is reduced. Standard Reduction Potential Vanadium.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential Vanadium This section looks at ways of changing between them. In this lab, we will be employing zinc, tin, and silver metals to reduce vanadium. The values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for the following. It starts with a bit of description, and. All other species are. Standard Reduction Potential Vanadium.
From www.slideserve.com
PPT The oxidation states of vanadium PowerPoint Presentation, free Standard Reduction Potential Vanadium Vanadium is reduced from an oxidation number of +3 to +2 in half equation 3. All other species are aqueous. Based on your results and the standard reduction potential of each addition, you should be able to. In this lab, we will be employing zinc, tin, and silver metals to reduce vanadium. Solids, gases, and liquids are identified; Reduction reactions. Standard Reduction Potential Vanadium.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Standard Reduction Potential Vanadium Zn is the best reducing agent. The different oxidation states of vanadium are evident due to color changes, and can be separated by. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Vanadium has oxidation states in its compounds of +5, +4, +3 and +2. All other species are. Standard Reduction Potential Vanadium.
From www.researchgate.net
Redox potentials and type of process for the V IV /V IV O species Standard Reduction Potential Vanadium Reduction reactions in acidic solution are written using h + in place of. Values are from the following sources: Solids, gases, and liquids are identified; Vanadium is reduced from an oxidation number of +3 to +2 in half equation 3. In this lab, v5+ is reduced to v4+, v3+, v2+. Vanadium has oxidation states in its compounds of +5, +4,. Standard Reduction Potential Vanadium.
From www.slideserve.com
PPT The Oxidation States of Vanadium PowerPoint Presentation, free Standard Reduction Potential Vanadium The values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for the following. In this lab, v5+ is reduced to v4+, v3+, v2+. Zn is the best reducing agent. Values are from the following sources: Based on your results and the standard reduction potential of each addition, you should. Standard Reduction Potential Vanadium.
From ch302.cm.utexas.edu
Electrochemistry_Reduction_Potentials Standard Reduction Potential Vanadium All other species are aqueous. Reduction reactions in acidic solution are written using h + in place of. Vanadium has oxidation states in its compounds of +5, +4, +3 and +2. The values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for the following. Values are from the following. Standard Reduction Potential Vanadium.
From www.numerade.com
SOLVEDUse data from the text to construct a standard electrode Standard Reduction Potential Vanadium In this lab, v5+ is reduced to v4+, v3+, v2+. Vanadium is reduced from an oxidation number of +3 to +2 in half equation 3. Based on your results and the standard reduction potential of each addition, you should be able to. The following table provides e o for selected reduction reactions. Solids, gases, and liquids are identified; Reduction reactions. Standard Reduction Potential Vanadium.
From www.slideserve.com
PPT The Oxidation States of Vanadium PowerPoint Presentation, free Standard Reduction Potential Vanadium Vanadium has oxidation states in its compounds of +5, +4, +3 and +2. In this lab, we will be employing zinc, tin, and silver metals to reduce vanadium. Reduction reactions in acidic solution are written using h + in place of. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she),. Standard Reduction Potential Vanadium.
From f15.beauty
Standard Reduction Potential Table Standard Reduction Potential Vanadium It starts with a bit of description, and. Zn is the best reducing agent. In this lab, we will be employing zinc, tin, and silver metals to reduce vanadium. The e θ value for half equation 2 is more negative than the e θ for half equation 3. Solids, gases, and liquids are identified; Vanadium is reduced from an oxidation. Standard Reduction Potential Vanadium.
From testbook.com
Reduction Potential Measurement, Half Cells, Differences & More Standard Reduction Potential Vanadium In this lab, we will be employing zinc, tin, and silver metals to reduce vanadium. Vanadium is reduced from an oxidation number of +3 to +2 in half equation 3. Vanadium has oxidation states in its compounds of +5, +4, +3 and +2. The different oxidation states of vanadium are evident due to color changes, and can be separated by.. Standard Reduction Potential Vanadium.
From mavink.com
Standard Reduction Potentials Standard Reduction Potential Vanadium The values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for the following. In this lab, we will be employing zinc, tin, and silver metals to reduce vanadium. Vanadium has oxidation states in its compounds of +5, +4, +3 and +2. It starts with a bit of description, and.. Standard Reduction Potential Vanadium.
From www.pveducation.org
Standard Potential PVEducation Standard Reduction Potential Vanadium Reduction reactions in acidic solution are written using h + in place of. Zn is the best reducing agent. Vanadium is reduced from an oxidation number of +3 to +2 in half equation 3. Solids, gases, and liquids are identified; Vanadium has oxidation states in its compounds of +5, +4, +3 and +2. All other species are aqueous. This section. Standard Reduction Potential Vanadium.
From www.slideserve.com
PPT The Oxidation States of Vanadium PowerPoint Presentation ID4176570 Standard Reduction Potential Vanadium Based on your results and the standard reduction potential of each addition, you should be able to. Values are from the following sources: All other species are aqueous. This section looks at ways of changing between them. Vanadium has oxidation states in its compounds of +5, +4, +3 and +2. Solids, gases, and liquids are identified; In this lab, v5+. Standard Reduction Potential Vanadium.
From chempedia.info
Vanadium potential diagram Big Chemical Encyclopedia Standard Reduction Potential Vanadium This section looks at ways of changing between them. The e θ value for half equation 2 is more negative than the e θ for half equation 3. In this lab, we will be employing zinc, tin, and silver metals to reduce vanadium. In this lab, v5+ is reduced to v4+, v3+, v2+. Values are from the following sources: All. Standard Reduction Potential Vanadium.
From ch302.cm.utexas.edu
stdpotsshortlist.png Standard Reduction Potential Vanadium Based on your results and the standard reduction potential of each addition, you should be able to. Vanadium has oxidation states in its compounds of +5, +4, +3 and +2. Zn is the best reducing agent. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The different oxidation states. Standard Reduction Potential Vanadium.
From jonathancoles.z13.web.core.windows.net
Standard Reduction Potential Chart Standard Reduction Potential Vanadium It starts with a bit of description, and. In this lab, v5+ is reduced to v4+, v3+, v2+. The values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for the following. Values are from the following sources: In this lab, we will be employing zinc, tin, and silver metals. Standard Reduction Potential Vanadium.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Vanadium Reduction reactions in acidic solution are written using h + in place of. Solids, gases, and liquids are identified; The e θ value for half equation 2 is more negative than the e θ for half equation 3. Vanadium has oxidation states in its compounds of +5, +4, +3 and +2. This section looks at ways of changing between them.. Standard Reduction Potential Vanadium.
From chempedia.info
Vanadium potential diagram Big Chemical Encyclopedia Standard Reduction Potential Vanadium This section looks at ways of changing between them. Vanadium is reduced from an oxidation number of +3 to +2 in half equation 3. In this lab, v5+ is reduced to v4+, v3+, v2+. Based on your results and the standard reduction potential of each addition, you should be able to. The different oxidation states of vanadium are evident due. Standard Reduction Potential Vanadium.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Vanadium The e θ value for half equation 2 is more negative than the e θ for half equation 3. All other species are aqueous. The values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for the following. Vanadium has oxidation states in its compounds of +5, +4, +3 and. Standard Reduction Potential Vanadium.
From www.researchgate.net
Oxidation and Reduction Potentials of the Vanadium(V)NHC Complexes 1 Standard Reduction Potential Vanadium This section looks at ways of changing between them. In this lab, we will be employing zinc, tin, and silver metals to reduce vanadium. Based on your results and the standard reduction potential of each addition, you should be able to. In this lab, v5+ is reduced to v4+, v3+, v2+. Zn is the best reducing agent. Solids, gases, and. Standard Reduction Potential Vanadium.
From www.docbrown.info
Vanadium transition metal Chemistry ions vanadium(II) V2+ vanadium(III Standard Reduction Potential Vanadium This section looks at ways of changing between them. In this lab, we will be employing zinc, tin, and silver metals to reduce vanadium. Values are from the following sources: The values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for the following. All other species are aqueous. The. Standard Reduction Potential Vanadium.
From www.flinnsci.ca
Standard Reduction Potential Charts for Chemistry Standard Reduction Potential Vanadium The following table provides e o for selected reduction reactions. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Vanadium is reduced from an oxidation number of +3 to +2 in half equation 3. In this lab, we will be employing zinc, tin, and silver metals to reduce vanadium.. Standard Reduction Potential Vanadium.
From www.slideserve.com
PPT The oxidation states of vanadium PowerPoint Presentation ID467926 Standard Reduction Potential Vanadium The different oxidation states of vanadium are evident due to color changes, and can be separated by. In this lab, v5+ is reduced to v4+, v3+, v2+. It starts with a bit of description, and. The following table provides e o for selected reduction reactions. In this lab, we will be employing zinc, tin, and silver metals to reduce vanadium.. Standard Reduction Potential Vanadium.
From www.youtube.com
Further reduction of Vanadium Electrode potential Challenge (A Level Standard Reduction Potential Vanadium The different oxidation states of vanadium are evident due to color changes, and can be separated by. The values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for the following. In this lab, we will be employing zinc, tin, and silver metals to reduce vanadium. Values are from the. Standard Reduction Potential Vanadium.
From edu.rsc.org
The oxidation states of vanadium Experiment RSC Education Standard Reduction Potential Vanadium Values are from the following sources: In this lab, v5+ is reduced to v4+, v3+, v2+. Reduction reactions in acidic solution are written using h + in place of. Solids, gases, and liquids are identified; The different oxidation states of vanadium are evident due to color changes, and can be separated by. Based on your results and the standard reduction. Standard Reduction Potential Vanadium.
From www.slideserve.com
PPT The oxidation states of vanadium PowerPoint Presentation, free Standard Reduction Potential Vanadium The different oxidation states of vanadium are evident due to color changes, and can be separated by. The values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for the following. This section looks at ways of changing between them. The following table provides e o for selected reduction reactions.. Standard Reduction Potential Vanadium.
From www.slideserve.com
PPT Electrochemistry PowerPoint Presentation, free download ID4831905 Standard Reduction Potential Vanadium Zn is the best reducing agent. 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. Based on your results and the standard reduction potential of each addition, you should be able to. Solids, gases, and liquids are identified; In this lab, we will be employing zinc, tin, and silver. Standard Reduction Potential Vanadium.
From www.researchgate.net
Speciation diagram showing the oxidation state and major species of Standard Reduction Potential Vanadium Zn is the best reducing agent. In this lab, we will be employing zinc, tin, and silver metals to reduce vanadium. Solids, gases, and liquids are identified; 372 rows the data below tabulates standard electrode potentials (e°), in volts relative to the standard hydrogen electrode (she), at:. The e θ value for half equation 2 is more negative than the. Standard Reduction Potential Vanadium.
From www.slideserve.com
PPT The oxidation states of vanadium PowerPoint Presentation, free Standard Reduction Potential Vanadium This section looks at ways of changing between them. Based on your results and the standard reduction potential of each addition, you should be able to. The following table provides e o for selected reduction reactions. The different oxidation states of vanadium are evident due to color changes, and can be separated by. Reduction reactions in acidic solution are written. Standard Reduction Potential Vanadium.
From www.slideserve.com
PPT The Oxidation States of Vanadium PowerPoint Presentation, free Standard Reduction Potential Vanadium Values are from the following sources: The values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for the following. The different oxidation states of vanadium are evident due to color changes, and can be separated by. Reduction reactions in acidic solution are written using h + in place of.. Standard Reduction Potential Vanadium.
From www.numerade.com
SOLVED Given the following table of standard reduction potentials for Standard Reduction Potential Vanadium Zn is the best reducing agent. The values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for the following. The following table provides e o for selected reduction reactions. Vanadium is reduced from an oxidation number of +3 to +2 in half equation 3. In this lab, we will. Standard Reduction Potential Vanadium.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Vanadium The e θ value for half equation 2 is more negative than the e θ for half equation 3. Solids, gases, and liquids are identified; All other species are aqueous. Reduction reactions in acidic solution are written using h + in place of. This section looks at ways of changing between them. 372 rows the data below tabulates standard electrode. Standard Reduction Potential Vanadium.
From mungfali.com
Standard Reduction Potentials Table Standard Reduction Potential Vanadium Vanadium is reduced from an oxidation number of +3 to +2 in half equation 3. Values are from the following sources: Solids, gases, and liquids are identified; It starts with a bit of description, and. All other species are aqueous. In this lab, we will be employing zinc, tin, and silver metals to reduce vanadium. Reduction reactions in acidic solution. Standard Reduction Potential Vanadium.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells Standard Reduction Potential Vanadium Solids, gases, and liquids are identified; The values of standard electrode potentials are given in the table in volts relative to the standard hydrogen electrode and are for the following. Values are from the following sources: Based on your results and the standard reduction potential of each addition, you should be able to. The e θ value for half equation. Standard Reduction Potential Vanadium.