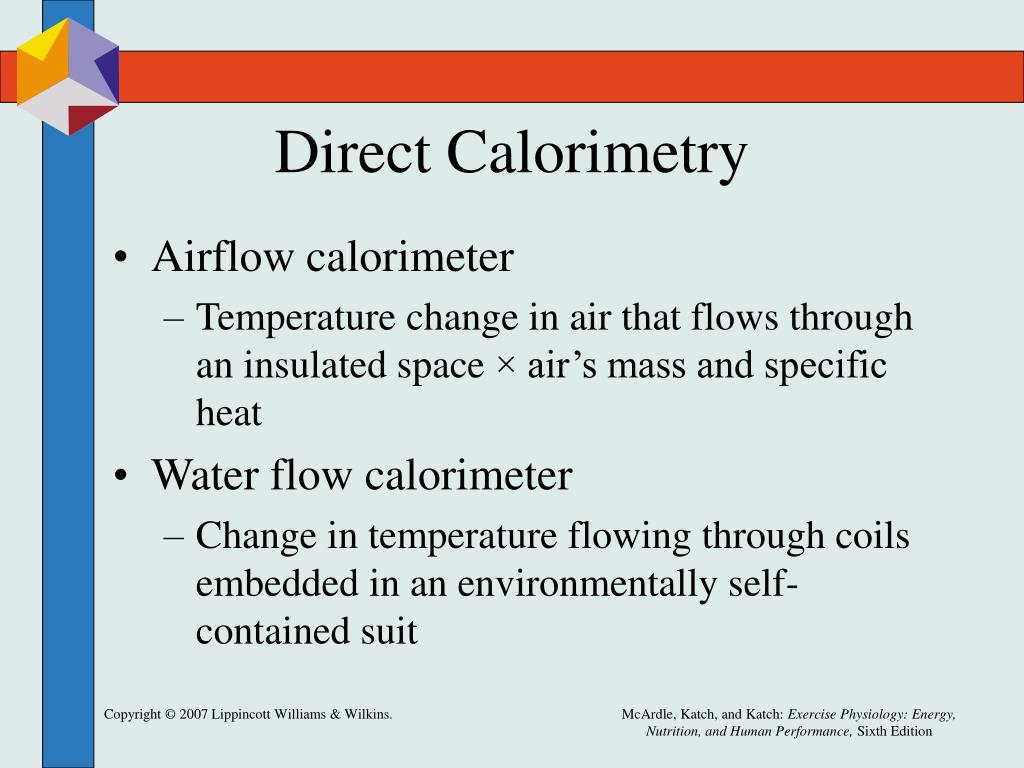
Define Calorimetry Direct . direct calorimetry is the gold standard means of measuring human metabolic rate and its use has been fundamental for. direct calorimetry obtains a direct measurement of the amount of heat generated by the body within a structure. — direct calorimetry: It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. — direct calorimetry. direct calorimetry provides a measure of energy expended in the form of heat. this chapter describes calorimetry or the direct measurement of heat production, a technique first used by lavoisier and. The direct calorimetry technique measures the rate of heat loss by the subject using a. direct calorimetry, measuring the heat produced by the flame directly, is the most obvious and accurate method. The subject is placed in an insulated chamber.
from www.slideserve.com
in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. this chapter describes calorimetry or the direct measurement of heat production, a technique first used by lavoisier and. The subject is placed in an insulated chamber. direct calorimetry, measuring the heat produced by the flame directly, is the most obvious and accurate method. direct calorimetry obtains a direct measurement of the amount of heat generated by the body within a structure. direct calorimetry is the gold standard means of measuring human metabolic rate and its use has been fundamental for. direct calorimetry provides a measure of energy expended in the form of heat. — direct calorimetry. It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. — direct calorimetry:
PPT Chapter 8 PowerPoint Presentation, free download ID5579931
Define Calorimetry Direct The subject is placed in an insulated chamber. direct calorimetry obtains a direct measurement of the amount of heat generated by the body within a structure. direct calorimetry, measuring the heat produced by the flame directly, is the most obvious and accurate method. It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. direct calorimetry is the gold standard means of measuring human metabolic rate and its use has been fundamental for. The direct calorimetry technique measures the rate of heat loss by the subject using a. — direct calorimetry. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. this chapter describes calorimetry or the direct measurement of heat production, a technique first used by lavoisier and. — direct calorimetry: The subject is placed in an insulated chamber. direct calorimetry provides a measure of energy expended in the form of heat.
From www.slideshare.net
Energy Define Calorimetry Direct direct calorimetry obtains a direct measurement of the amount of heat generated by the body within a structure. direct calorimetry provides a measure of energy expended in the form of heat. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. direct calorimetry, measuring the heat produced by the flame. Define Calorimetry Direct.
From www.thoughtco.com
Calorimeter Definition in Chemistry Define Calorimetry Direct The subject is placed in an insulated chamber. direct calorimetry is the gold standard means of measuring human metabolic rate and its use has been fundamental for. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. — direct calorimetry. The direct calorimetry technique measures the rate of heat loss by. Define Calorimetry Direct.
From chemicalada.blogspot.com
MODES OF HEAT TRNSFER Define Calorimetry Direct The direct calorimetry technique measures the rate of heat loss by the subject using a. direct calorimetry provides a measure of energy expended in the form of heat. — direct calorimetry: The subject is placed in an insulated chamber. It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. in. Define Calorimetry Direct.
From www.youtube.com
Calorimetry calculation YouTube Define Calorimetry Direct in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. direct calorimetry is the gold standard means of measuring human metabolic rate and its use has been fundamental for. direct calorimetry provides a measure of energy expended in the form of heat. direct calorimetry obtains a direct measurement of the. Define Calorimetry Direct.
From chemwiki.ucdavis.edu
Chapter 9.6 Calorimetry Chemwiki Define Calorimetry Direct — direct calorimetry: this chapter describes calorimetry or the direct measurement of heat production, a technique first used by lavoisier and. The direct calorimetry technique measures the rate of heat loss by the subject using a. direct calorimetry obtains a direct measurement of the amount of heat generated by the body within a structure. direct calorimetry. Define Calorimetry Direct.
From www.youtube.com
Numericals of Calorimetry Calorimetry Class 10 Physics ICSE YouTube Define Calorimetry Direct The direct calorimetry technique measures the rate of heat loss by the subject using a. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. The subject is placed in an insulated chamber. direct calorimetry provides a measure of energy expended in the form of heat. direct calorimetry obtains a direct. Define Calorimetry Direct.
From www.collegesearch.in
Principle of Calorimetry Definition, Formula, Principle, Types Define Calorimetry Direct direct calorimetry, measuring the heat produced by the flame directly, is the most obvious and accurate method. direct calorimetry is the gold standard means of measuring human metabolic rate and its use has been fundamental for. direct calorimetry obtains a direct measurement of the amount of heat generated by the body within a structure. The direct calorimetry. Define Calorimetry Direct.
From www.slideshare.net
Energy Define Calorimetry Direct direct calorimetry is the gold standard means of measuring human metabolic rate and its use has been fundamental for. this chapter describes calorimetry or the direct measurement of heat production, a technique first used by lavoisier and. direct calorimetry obtains a direct measurement of the amount of heat generated by the body within a structure. The subject. Define Calorimetry Direct.
From www.slideserve.com
PPT Measurement of Energy in Food and During Physical Activity Define Calorimetry Direct It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. — direct calorimetry. direct calorimetry is the gold standard means of measuring human metabolic rate and its use has been fundamental for. The subject is placed in an insulated chamber. — direct calorimetry: direct calorimetry, measuring the heat produced. Define Calorimetry Direct.
From thechemistrynotes.com
Calorimetry Definition, Principle, Types, Application, and Limitations Define Calorimetry Direct direct calorimetry obtains a direct measurement of the amount of heat generated by the body within a structure. direct calorimetry, measuring the heat produced by the flame directly, is the most obvious and accurate method. direct calorimetry provides a measure of energy expended in the form of heat. — direct calorimetry: The direct calorimetry technique measures. Define Calorimetry Direct.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation Define Calorimetry Direct It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. direct calorimetry obtains a direct measurement of the amount of heat generated by the body within a structure. The subject is placed in an insulated chamber. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is. Define Calorimetry Direct.
From www.chegg.com
Solved 1. Define direct calorimetry and indirect Define Calorimetry Direct — direct calorimetry: direct calorimetry is the gold standard means of measuring human metabolic rate and its use has been fundamental for. The direct calorimetry technique measures the rate of heat loss by the subject using a. — direct calorimetry. direct calorimetry provides a measure of energy expended in the form of heat. It detects the. Define Calorimetry Direct.
From glossary.periodni.com
Bomb calorimeter Chemistry Dictionary & Glossary Define Calorimetry Direct — direct calorimetry: in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. direct calorimetry, measuring the heat produced by the flame directly, is the most obvious and accurate method. this chapter describes calorimetry or the direct measurement of heat production, a technique first used by lavoisier and. direct. Define Calorimetry Direct.
From www.slideserve.com
PPT Chapter 8 PowerPoint Presentation, free download ID5579931 Define Calorimetry Direct The direct calorimetry technique measures the rate of heat loss by the subject using a. — direct calorimetry: this chapter describes calorimetry or the direct measurement of heat production, a technique first used by lavoisier and. — direct calorimetry. It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. . Define Calorimetry Direct.
From www.youtube.com
Principle of Calorimetry YouTube Define Calorimetry Direct direct calorimetry provides a measure of energy expended in the form of heat. direct calorimetry obtains a direct measurement of the amount of heat generated by the body within a structure. direct calorimetry, measuring the heat produced by the flame directly, is the most obvious and accurate method. — direct calorimetry. this chapter describes calorimetry. Define Calorimetry Direct.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Define Calorimetry Direct direct calorimetry, measuring the heat produced by the flame directly, is the most obvious and accurate method. direct calorimetry provides a measure of energy expended in the form of heat. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. It detects the heat change of a chemical reaction by directly. Define Calorimetry Direct.
From www.slideserve.com
PPT Energy Expenditure PowerPoint Presentation, free download ID Define Calorimetry Direct direct calorimetry is the gold standard means of measuring human metabolic rate and its use has been fundamental for. The subject is placed in an insulated chamber. It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. this chapter describes calorimetry or the direct measurement of heat production, a technique first. Define Calorimetry Direct.
From www.youtube.com
Calorimetry, Bomb Calorimetry, Constant Pressure Calorimetry FULL Define Calorimetry Direct this chapter describes calorimetry or the direct measurement of heat production, a technique first used by lavoisier and. It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. The subject is placed in an insulated chamber. direct calorimetry is the gold standard means of measuring human metabolic rate and its use. Define Calorimetry Direct.
From dxowceosf.blob.core.windows.net
Calorimetry All Formulas at Spencer McSwain blog Define Calorimetry Direct The subject is placed in an insulated chamber. direct calorimetry provides a measure of energy expended in the form of heat. — direct calorimetry. It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. direct calorimetry, measuring the heat produced by the flame directly, is the most obvious and accurate. Define Calorimetry Direct.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Define Calorimetry Direct direct calorimetry is the gold standard means of measuring human metabolic rate and its use has been fundamental for. — direct calorimetry. The subject is placed in an insulated chamber. The direct calorimetry technique measures the rate of heat loss by the subject using a. — direct calorimetry: this chapter describes calorimetry or the direct measurement. Define Calorimetry Direct.
From www.slideshare.net
Energy Define Calorimetry Direct this chapter describes calorimetry or the direct measurement of heat production, a technique first used by lavoisier and. The direct calorimetry technique measures the rate of heat loss by the subject using a. direct calorimetry, measuring the heat produced by the flame directly, is the most obvious and accurate method. — direct calorimetry: It detects the heat. Define Calorimetry Direct.
From www.numerade.com
SOLVED Describe indirect and direct calorimetry. What are the pros and Define Calorimetry Direct in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. this chapter describes calorimetry or the direct measurement of heat production, a technique first used by lavoisier and. — direct calorimetry: It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. direct calorimetry. Define Calorimetry Direct.
From www.slideserve.com
PPT Physiology, Health & Exercise PowerPoint Presentation, free Define Calorimetry Direct direct calorimetry provides a measure of energy expended in the form of heat. this chapter describes calorimetry or the direct measurement of heat production, a technique first used by lavoisier and. direct calorimetry, measuring the heat produced by the flame directly, is the most obvious and accurate method. — direct calorimetry: — direct calorimetry. The. Define Calorimetry Direct.
From gamma.app
Measurement of Energy Requirement by Direct Calorimetry Define Calorimetry Direct The direct calorimetry technique measures the rate of heat loss by the subject using a. this chapter describes calorimetry or the direct measurement of heat production, a technique first used by lavoisier and. direct calorimetry obtains a direct measurement of the amount of heat generated by the body within a structure. direct calorimetry is the gold standard. Define Calorimetry Direct.
From www.sliderbase.com
Basic Thermochemistry Presentation Chemistry Define Calorimetry Direct in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. direct calorimetry is the gold standard means of measuring human metabolic rate and its use has been fundamental for. direct calorimetry, measuring the heat produced by the flame directly, is the most obvious and accurate method. direct calorimetry provides a. Define Calorimetry Direct.
From www.studocu.com
Calorimetry chemistry CALORIMETRY Learning Objectives Define Define Calorimetry Direct The subject is placed in an insulated chamber. this chapter describes calorimetry or the direct measurement of heat production, a technique first used by lavoisier and. direct calorimetry obtains a direct measurement of the amount of heat generated by the body within a structure. — direct calorimetry: in chemistry and thermodynamics, calorimetry (from latin calor 'heat'. Define Calorimetry Direct.
From thechemistrynotes.com
Calorimeter Definition, Types and Uses Define Calorimetry Direct It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. — direct calorimetry. direct calorimetry obtains a direct measurement of the amount of heat generated by the body within a structure. — direct calorimetry: direct calorimetry is the gold standard means of measuring human metabolic rate and its use. Define Calorimetry Direct.
From users.highland.edu
Calorimetry Define Calorimetry Direct The subject is placed in an insulated chamber. direct calorimetry, measuring the heat produced by the flame directly, is the most obvious and accurate method. direct calorimetry is the gold standard means of measuring human metabolic rate and its use has been fundamental for. — direct calorimetry. in chemistry and thermodynamics, calorimetry (from latin calor 'heat'. Define Calorimetry Direct.
From chem.libretexts.org
11.5 Reaction Calorimetry Chemistry LibreTexts Define Calorimetry Direct The direct calorimetry technique measures the rate of heat loss by the subject using a. direct calorimetry provides a measure of energy expended in the form of heat. The subject is placed in an insulated chamber. direct calorimetry, measuring the heat produced by the flame directly, is the most obvious and accurate method. this chapter describes calorimetry. Define Calorimetry Direct.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Define Calorimetry Direct The direct calorimetry technique measures the rate of heat loss by the subject using a. — direct calorimetry: — direct calorimetry. direct calorimetry, measuring the heat produced by the flame directly, is the most obvious and accurate method. direct calorimetry is the gold standard means of measuring human metabolic rate and its use has been fundamental. Define Calorimetry Direct.
From punchlistzero.com
Is Heat a State Function? Punchlist Zero Define Calorimetry Direct direct calorimetry provides a measure of energy expended in the form of heat. — direct calorimetry. It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. direct calorimetry is the gold standard means of measuring human metabolic rate and its use has been fundamental for. The direct calorimetry technique measures. Define Calorimetry Direct.
From www.studypool.com
SOLUTION Direct calorimetry and indirect calorimetry Studypool Define Calorimetry Direct direct calorimetry, measuring the heat produced by the flame directly, is the most obvious and accurate method. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. — direct calorimetry: this chapter describes calorimetry or the direct measurement of heat production, a technique first used by lavoisier and. It detects. Define Calorimetry Direct.
From www.youtube.com
BASIC PRINCIPLE OF CALORIMETRY YouTube Define Calorimetry Direct — direct calorimetry. It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. The subject is placed in an insulated chamber. direct calorimetry obtains a direct measurement of the amount of heat generated by the body within a structure. — direct calorimetry: direct calorimetry provides a measure of energy. Define Calorimetry Direct.
From www.numerade.com
SOLVEDThe defining equation for calorimetry is Define Calorimetry Direct direct calorimetry provides a measure of energy expended in the form of heat. direct calorimetry is the gold standard means of measuring human metabolic rate and its use has been fundamental for. in chemistry and thermodynamics, calorimetry (from latin calor 'heat' and greek μέτρον (metron) 'measure') is the. The direct calorimetry technique measures the rate of heat. Define Calorimetry Direct.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson Define Calorimetry Direct — direct calorimetry: — direct calorimetry. The direct calorimetry technique measures the rate of heat loss by the subject using a. direct calorimetry obtains a direct measurement of the amount of heat generated by the body within a structure. It detects the heat change of a chemical reaction by directly measuring the temperature change it creates. . Define Calorimetry Direct.