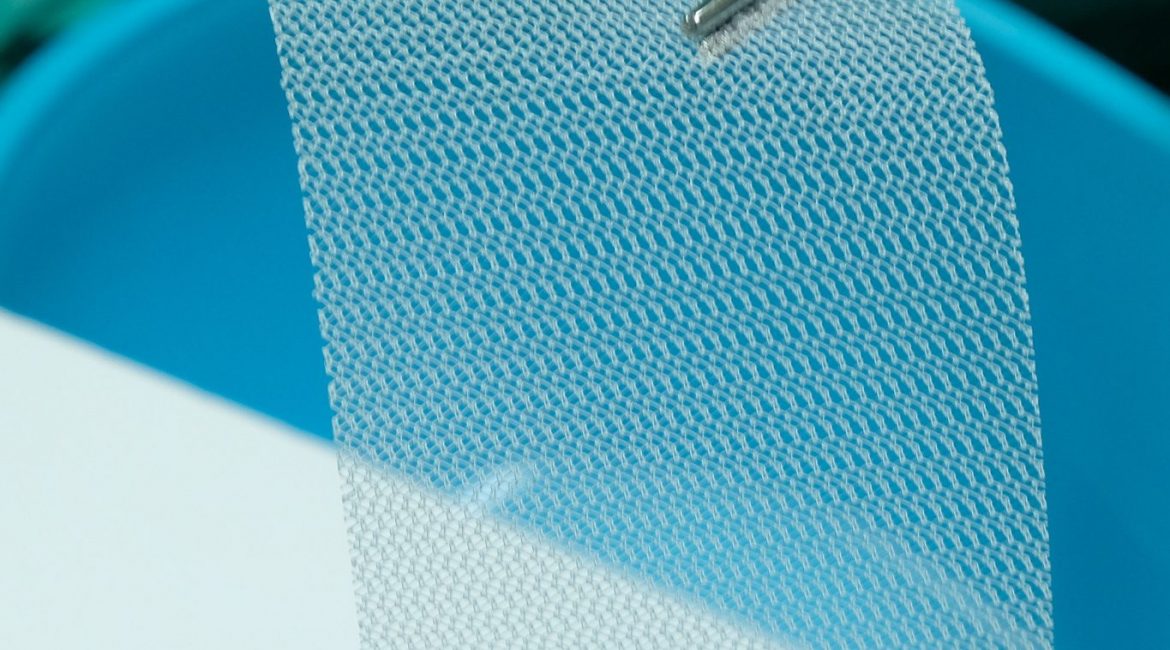
Medical Mesh Recall . This leads to recalls of specific devices, including johnson & johnson’s ethicon physiomesh in 2016. Learn about the latest hernia mesh recalls, potential complications and what to do if your hernia mesh has been recalled. Court documents don’t specify an exact settlement amount, but they state that the parties agreed to dismiss the cases and bear their own costs. As a result of some of the complications arising from these surgical meshes, the fda has recalled a variety of hernia mesh implants over the years. Hernia mesh lawsuits are legal claims against hernia mesh manufacturers filed by people who suffered serious injuries after their hernia mesh implants failed. As of october 2024, over 25,000 hernia mesh lawsuits remain pending across multiple mdls. Plaintiffs claimed defects in ethicon’s physiomesh flexible composite hernia mesh led them to develop complications such as recurrent hernias, mesh migration, infection, bleeding and pain. From 2005 through 2021, hernia mesh manufacturers recalled more than 211,000 units of hernia mesh. Open repair that uses sutures (stitches) without mesh is referred to. Open repair can be done with or without surgical mesh.
from www.druganddevicewatch.com
Hernia mesh lawsuits are legal claims against hernia mesh manufacturers filed by people who suffered serious injuries after their hernia mesh implants failed. As a result of some of the complications arising from these surgical meshes, the fda has recalled a variety of hernia mesh implants over the years. Open repair can be done with or without surgical mesh. From 2005 through 2021, hernia mesh manufacturers recalled more than 211,000 units of hernia mesh. This leads to recalls of specific devices, including johnson & johnson’s ethicon physiomesh in 2016. Plaintiffs claimed defects in ethicon’s physiomesh flexible composite hernia mesh led them to develop complications such as recurrent hernias, mesh migration, infection, bleeding and pain. Open repair that uses sutures (stitches) without mesh is referred to. As of october 2024, over 25,000 hernia mesh lawsuits remain pending across multiple mdls. Learn about the latest hernia mesh recalls, potential complications and what to do if your hernia mesh has been recalled. Court documents don’t specify an exact settlement amount, but they state that the parties agreed to dismiss the cases and bear their own costs.
Bard Hernia Mesh Linked to Injuries Drug And Device Watch
Medical Mesh Recall Plaintiffs claimed defects in ethicon’s physiomesh flexible composite hernia mesh led them to develop complications such as recurrent hernias, mesh migration, infection, bleeding and pain. Learn about the latest hernia mesh recalls, potential complications and what to do if your hernia mesh has been recalled. Open repair that uses sutures (stitches) without mesh is referred to. This leads to recalls of specific devices, including johnson & johnson’s ethicon physiomesh in 2016. From 2005 through 2021, hernia mesh manufacturers recalled more than 211,000 units of hernia mesh. Plaintiffs claimed defects in ethicon’s physiomesh flexible composite hernia mesh led them to develop complications such as recurrent hernias, mesh migration, infection, bleeding and pain. As of october 2024, over 25,000 hernia mesh lawsuits remain pending across multiple mdls. As a result of some of the complications arising from these surgical meshes, the fda has recalled a variety of hernia mesh implants over the years. Court documents don’t specify an exact settlement amount, but they state that the parties agreed to dismiss the cases and bear their own costs. Hernia mesh lawsuits are legal claims against hernia mesh manufacturers filed by people who suffered serious injuries after their hernia mesh implants failed. Open repair can be done with or without surgical mesh.
From www.pinterest.com
Pin on HerniaMeshLawsuit Medical Mesh Recall As of october 2024, over 25,000 hernia mesh lawsuits remain pending across multiple mdls. Open repair that uses sutures (stitches) without mesh is referred to. From 2005 through 2021, hernia mesh manufacturers recalled more than 211,000 units of hernia mesh. As a result of some of the complications arising from these surgical meshes, the fda has recalled a variety of. Medical Mesh Recall.
From www.kugelherniameshclassaction.com
Marlex Hernia Mesh Lawsuit Manufactured by C.R. Bard Medical Mesh Recall Plaintiffs claimed defects in ethicon’s physiomesh flexible composite hernia mesh led them to develop complications such as recurrent hernias, mesh migration, infection, bleeding and pain. As of october 2024, over 25,000 hernia mesh lawsuits remain pending across multiple mdls. Open repair that uses sutures (stitches) without mesh is referred to. As a result of some of the complications arising from. Medical Mesh Recall.
From list.ly
Hernia Mesh Recall A Listly List Medical Mesh Recall Court documents don’t specify an exact settlement amount, but they state that the parties agreed to dismiss the cases and bear their own costs. Plaintiffs claimed defects in ethicon’s physiomesh flexible composite hernia mesh led them to develop complications such as recurrent hernias, mesh migration, infection, bleeding and pain. Hernia mesh lawsuits are legal claims against hernia mesh manufacturers filed. Medical Mesh Recall.
From www.youtube.com
Hernia Mesh Recall YouTube Medical Mesh Recall Open repair can be done with or without surgical mesh. Learn about the latest hernia mesh recalls, potential complications and what to do if your hernia mesh has been recalled. From 2005 through 2021, hernia mesh manufacturers recalled more than 211,000 units of hernia mesh. Hernia mesh lawsuits are legal claims against hernia mesh manufacturers filed by people who suffered. Medical Mesh Recall.
From www.youtube.com
Vaginal Mesh Complications Explained YouTube Medical Mesh Recall As a result of some of the complications arising from these surgical meshes, the fda has recalled a variety of hernia mesh implants over the years. As of october 2024, over 25,000 hernia mesh lawsuits remain pending across multiple mdls. Hernia mesh lawsuits are legal claims against hernia mesh manufacturers filed by people who suffered serious injuries after their hernia. Medical Mesh Recall.
From herniameshrecall.wordpress.com
Get Legal Help On Hernia Mesh Complications Hernia Mesh Recall Medical Mesh Recall Court documents don’t specify an exact settlement amount, but they state that the parties agreed to dismiss the cases and bear their own costs. As a result of some of the complications arising from these surgical meshes, the fda has recalled a variety of hernia mesh implants over the years. Open repair can be done with or without surgical mesh.. Medical Mesh Recall.
From www.chanfraulaw.com
Filing a Lawsuit for Defective Hernia Mesh Hernia Mesh Lawsuit Day... Medical Mesh Recall Hernia mesh lawsuits are legal claims against hernia mesh manufacturers filed by people who suffered serious injuries after their hernia mesh implants failed. As a result of some of the complications arising from these surgical meshes, the fda has recalled a variety of hernia mesh implants over the years. This leads to recalls of specific devices, including johnson & johnson’s. Medical Mesh Recall.
From www.herniameshpatchlawsuit.com
Hernia Mesh Recall Medical Mesh Recall Court documents don’t specify an exact settlement amount, but they state that the parties agreed to dismiss the cases and bear their own costs. From 2005 through 2021, hernia mesh manufacturers recalled more than 211,000 units of hernia mesh. Hernia mesh lawsuits are legal claims against hernia mesh manufacturers filed by people who suffered serious injuries after their hernia mesh. Medical Mesh Recall.
From www.sugarman.com
Hernia Mesh Failures and Injuries » Sugarman Medical Mesh Recall Court documents don’t specify an exact settlement amount, but they state that the parties agreed to dismiss the cases and bear their own costs. As of october 2024, over 25,000 hernia mesh lawsuits remain pending across multiple mdls. As a result of some of the complications arising from these surgical meshes, the fda has recalled a variety of hernia mesh. Medical Mesh Recall.
From www.personalinjurylawcal.com
Hernia Mesh Lawsuit Bard, Ethicon, Covidien & Atrium CQur Recalls Medical Mesh Recall Open repair that uses sutures (stitches) without mesh is referred to. Plaintiffs claimed defects in ethicon’s physiomesh flexible composite hernia mesh led them to develop complications such as recurrent hernias, mesh migration, infection, bleeding and pain. As of october 2024, over 25,000 hernia mesh lawsuits remain pending across multiple mdls. From 2005 through 2021, hernia mesh manufacturers recalled more than. Medical Mesh Recall.
From meshproblems.weebly.com
Links to Medical Mesh Research Links to Medical Mesh Research Medical Mesh Recall This leads to recalls of specific devices, including johnson & johnson’s ethicon physiomesh in 2016. Court documents don’t specify an exact settlement amount, but they state that the parties agreed to dismiss the cases and bear their own costs. Learn about the latest hernia mesh recalls, potential complications and what to do if your hernia mesh has been recalled. As. Medical Mesh Recall.
From www.academia.edu
(PDF) Patient recall 6 weeks after surgical consent for midurethral Medical Mesh Recall Hernia mesh lawsuits are legal claims against hernia mesh manufacturers filed by people who suffered serious injuries after their hernia mesh implants failed. Open repair can be done with or without surgical mesh. As a result of some of the complications arising from these surgical meshes, the fda has recalled a variety of hernia mesh implants over the years. Learn. Medical Mesh Recall.
From www.druganddevicewatch.com
Bard Hernia Mesh Linked to Injuries Drug And Device Watch Medical Mesh Recall Open repair can be done with or without surgical mesh. Open repair that uses sutures (stitches) without mesh is referred to. Hernia mesh lawsuits are legal claims against hernia mesh manufacturers filed by people who suffered serious injuries after their hernia mesh implants failed. From 2005 through 2021, hernia mesh manufacturers recalled more than 211,000 units of hernia mesh. Plaintiffs. Medical Mesh Recall.
From www.rnz.co.nz
Australian surgical mesh lawsuit begins RNZ News Medical Mesh Recall As a result of some of the complications arising from these surgical meshes, the fda has recalled a variety of hernia mesh implants over the years. This leads to recalls of specific devices, including johnson & johnson’s ethicon physiomesh in 2016. Open repair that uses sutures (stitches) without mesh is referred to. Hernia mesh lawsuits are legal claims against hernia. Medical Mesh Recall.
From www.wolfedlund.com
Dangers and Complications from Surgical Mesh Products Medical Mesh Recall Open repair can be done with or without surgical mesh. From 2005 through 2021, hernia mesh manufacturers recalled more than 211,000 units of hernia mesh. This leads to recalls of specific devices, including johnson & johnson’s ethicon physiomesh in 2016. As a result of some of the complications arising from these surgical meshes, the fda has recalled a variety of. Medical Mesh Recall.
From issuu.com
Surgical mesh lawsuit by surgicalmesh lawsuit Issuu Medical Mesh Recall Court documents don’t specify an exact settlement amount, but they state that the parties agreed to dismiss the cases and bear their own costs. This leads to recalls of specific devices, including johnson & johnson’s ethicon physiomesh in 2016. As a result of some of the complications arising from these surgical meshes, the fda has recalled a variety of hernia. Medical Mesh Recall.
From mblynchfirm.com
Ethicon’s Physiomesh Flexible Composite Mesh Recall Medical Mesh Recall Learn about the latest hernia mesh recalls, potential complications and what to do if your hernia mesh has been recalled. Open repair that uses sutures (stitches) without mesh is referred to. This leads to recalls of specific devices, including johnson & johnson’s ethicon physiomesh in 2016. From 2005 through 2021, hernia mesh manufacturers recalled more than 211,000 units of hernia. Medical Mesh Recall.
From laptrinhx.com
Hernia mesh lawsuit complications, failure & recall LaptrinhX / News Medical Mesh Recall As a result of some of the complications arising from these surgical meshes, the fda has recalled a variety of hernia mesh implants over the years. This leads to recalls of specific devices, including johnson & johnson’s ethicon physiomesh in 2016. Open repair can be done with or without surgical mesh. As of october 2024, over 25,000 hernia mesh lawsuits. Medical Mesh Recall.
From www.drugwatcher.org
How to tell if hernia mesh failed? • Medical Mesh Recall As a result of some of the complications arising from these surgical meshes, the fda has recalled a variety of hernia mesh implants over the years. From 2005 through 2021, hernia mesh manufacturers recalled more than 211,000 units of hernia mesh. Hernia mesh lawsuits are legal claims against hernia mesh manufacturers filed by people who suffered serious injuries after their. Medical Mesh Recall.
From www.youhavealawyer.com
Hernia Mesh Lawyers Hernia Patch Recall Lawsuits Medical Mesh Recall Plaintiffs claimed defects in ethicon’s physiomesh flexible composite hernia mesh led them to develop complications such as recurrent hernias, mesh migration, infection, bleeding and pain. Learn about the latest hernia mesh recalls, potential complications and what to do if your hernia mesh has been recalled. From 2005 through 2021, hernia mesh manufacturers recalled more than 211,000 units of hernia mesh.. Medical Mesh Recall.
From www.robertslawfirm.com
Recalled Hernia Mesh Complications • Roberts & Roberts Medical Mesh Recall As of october 2024, over 25,000 hernia mesh lawsuits remain pending across multiple mdls. Plaintiffs claimed defects in ethicon’s physiomesh flexible composite hernia mesh led them to develop complications such as recurrent hernias, mesh migration, infection, bleeding and pain. Learn about the latest hernia mesh recalls, potential complications and what to do if your hernia mesh has been recalled. From. Medical Mesh Recall.
From www.usrecallnews.com
Stronger Clinical Requirements Address Risks of Pelvic Organ Prolapse Medical Mesh Recall From 2005 through 2021, hernia mesh manufacturers recalled more than 211,000 units of hernia mesh. Open repair that uses sutures (stitches) without mesh is referred to. Hernia mesh lawsuits are legal claims against hernia mesh manufacturers filed by people who suffered serious injuries after their hernia mesh implants failed. Open repair can be done with or without surgical mesh. Court. Medical Mesh Recall.
From www.consumeralertnow.com
Hernia Mesh Lawsuits Hernia Mesh Settlements Consumer Alert Now Medical Mesh Recall As a result of some of the complications arising from these surgical meshes, the fda has recalled a variety of hernia mesh implants over the years. This leads to recalls of specific devices, including johnson & johnson’s ethicon physiomesh in 2016. Court documents don’t specify an exact settlement amount, but they state that the parties agreed to dismiss the cases. Medical Mesh Recall.
From list.ly
Hernia Mesh Recall A Listly List Medical Mesh Recall Plaintiffs claimed defects in ethicon’s physiomesh flexible composite hernia mesh led them to develop complications such as recurrent hernias, mesh migration, infection, bleeding and pain. Open repair can be done with or without surgical mesh. As a result of some of the complications arising from these surgical meshes, the fda has recalled a variety of hernia mesh implants over the. Medical Mesh Recall.
From www.firstelse.com
What You Should Know about a Hernia Mesh Recall First Else Medical Mesh Recall Plaintiffs claimed defects in ethicon’s physiomesh flexible composite hernia mesh led them to develop complications such as recurrent hernias, mesh migration, infection, bleeding and pain. Open repair can be done with or without surgical mesh. Hernia mesh lawsuits are legal claims against hernia mesh manufacturers filed by people who suffered serious injuries after their hernia mesh implants failed. This leads. Medical Mesh Recall.
From www.drugwatch.com
Ethicon Products, History, Mesh Recall and Lawsuits Medical Mesh Recall Hernia mesh lawsuits are legal claims against hernia mesh manufacturers filed by people who suffered serious injuries after their hernia mesh implants failed. Plaintiffs claimed defects in ethicon’s physiomesh flexible composite hernia mesh led them to develop complications such as recurrent hernias, mesh migration, infection, bleeding and pain. Learn about the latest hernia mesh recalls, potential complications and what to. Medical Mesh Recall.
From www.youtube.com
Transvaginal Mesh Lawsuit Lawyers YouTube Medical Mesh Recall Open repair that uses sutures (stitches) without mesh is referred to. Hernia mesh lawsuits are legal claims against hernia mesh manufacturers filed by people who suffered serious injuries after their hernia mesh implants failed. Plaintiffs claimed defects in ethicon’s physiomesh flexible composite hernia mesh led them to develop complications such as recurrent hernias, mesh migration, infection, bleeding and pain. As. Medical Mesh Recall.
From injurylawyer-news.com
Transvaginal Mesh Complications Surgical Mesh Problems, Failure Medical Mesh Recall This leads to recalls of specific devices, including johnson & johnson’s ethicon physiomesh in 2016. Court documents don’t specify an exact settlement amount, but they state that the parties agreed to dismiss the cases and bear their own costs. Hernia mesh lawsuits are legal claims against hernia mesh manufacturers filed by people who suffered serious injuries after their hernia mesh. Medical Mesh Recall.
From www.ctvnews.ca
Lawsuits being settled in surgical mesh complications CTV News Medical Mesh Recall Learn about the latest hernia mesh recalls, potential complications and what to do if your hernia mesh has been recalled. Hernia mesh lawsuits are legal claims against hernia mesh manufacturers filed by people who suffered serious injuries after their hernia mesh implants failed. This leads to recalls of specific devices, including johnson & johnson’s ethicon physiomesh in 2016. Open repair. Medical Mesh Recall.
From blog.factmr.com
Technical Advancements & Innovative Products Likely To Expand Medical Mesh Recall From 2005 through 2021, hernia mesh manufacturers recalled more than 211,000 units of hernia mesh. As of october 2024, over 25,000 hernia mesh lawsuits remain pending across multiple mdls. Learn about the latest hernia mesh recalls, potential complications and what to do if your hernia mesh has been recalled. As a result of some of the complications arising from these. Medical Mesh Recall.
From www.drugwatch.com
Transvaginal Mesh Complications Erosions & Organ Perforation Medical Mesh Recall Open repair that uses sutures (stitches) without mesh is referred to. Court documents don’t specify an exact settlement amount, but they state that the parties agreed to dismiss the cases and bear their own costs. From 2005 through 2021, hernia mesh manufacturers recalled more than 211,000 units of hernia mesh. Hernia mesh lawsuits are legal claims against hernia mesh manufacturers. Medical Mesh Recall.
From www.usrecallnews.com
Defective Atrium CQur Surgical Mesh US Recall News Medical Mesh Recall As of october 2024, over 25,000 hernia mesh lawsuits remain pending across multiple mdls. Learn about the latest hernia mesh recalls, potential complications and what to do if your hernia mesh has been recalled. As a result of some of the complications arising from these surgical meshes, the fda has recalled a variety of hernia mesh implants over the years.. Medical Mesh Recall.
From taylormartino.com
Defective Hernia Mesh Lawsuit Surgical Mesh Lawyers Medical Mesh Recall As of october 2024, over 25,000 hernia mesh lawsuits remain pending across multiple mdls. Hernia mesh lawsuits are legal claims against hernia mesh manufacturers filed by people who suffered serious injuries after their hernia mesh implants failed. From 2005 through 2021, hernia mesh manufacturers recalled more than 211,000 units of hernia mesh. Open repair that uses sutures (stitches) without mesh. Medical Mesh Recall.
From herniameshrecall.wordpress.com
Why Is The Hernia Mesh Surgery Causing You Pain? Hernia Mesh Recall Medical Mesh Recall From 2005 through 2021, hernia mesh manufacturers recalled more than 211,000 units of hernia mesh. Learn about the latest hernia mesh recalls, potential complications and what to do if your hernia mesh has been recalled. Hernia mesh lawsuits are legal claims against hernia mesh manufacturers filed by people who suffered serious injuries after their hernia mesh implants failed. Plaintiffs claimed. Medical Mesh Recall.
From herniameshme.wordpress.com
Scenario of Hernia Mesh Lawsuit Update Hernia Mesh.Me Medical Mesh Recall This leads to recalls of specific devices, including johnson & johnson’s ethicon physiomesh in 2016. Open repair can be done with or without surgical mesh. Plaintiffs claimed defects in ethicon’s physiomesh flexible composite hernia mesh led them to develop complications such as recurrent hernias, mesh migration, infection, bleeding and pain. As a result of some of the complications arising from. Medical Mesh Recall.