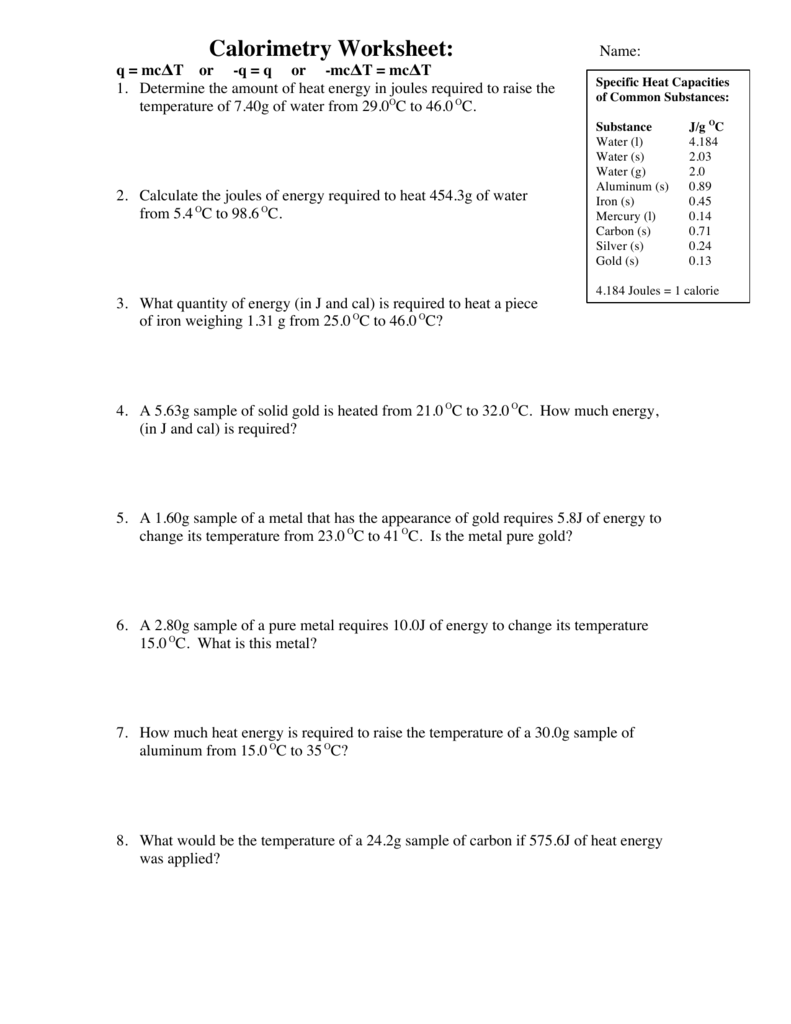
Calorimetry Packet Answers . 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the molar heat of combustion. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? When a substance is heated, the temperature of that substance increases. 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in joules) has it absorbed? What is the relationship between heat energy and temperature? Calorimeters can be used to find a substance’s specific heat. How do you think you can use the calorimeter to compare the specific heat capacities of the substances listed on the. Through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and to develop a deeper.
from www.abhayjere.com
How do you think you can use the calorimeter to compare the specific heat capacities of the substances listed on the. Calorimeters can be used to find a substance’s specific heat. When a substance is heated, the temperature of that substance increases. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the molar heat of combustion. 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in joules) has it absorbed? When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and to develop a deeper. What is the relationship between heat energy and temperature?
Calorimetry Worksheet Answer Key
Calorimetry Packet Answers When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. Calorimeters can be used to find a substance’s specific heat. Through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and to develop a deeper. How do you think you can use the calorimeter to compare the specific heat capacities of the substances listed on the. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in joules) has it absorbed? 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the molar heat of combustion. When a substance is heated, the temperature of that substance increases. What is the relationship between heat energy and temperature?
From www.scribd.com
Exercise 6.2b Calorimetry Answers PDF Calorimetry Packet Answers 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in joules) has it absorbed? How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? What is the relationship between heat energy and temperature? When a hot object is placed in the. Calorimetry Packet Answers.
From sweetnowsw.blogspot.com
Calorimetry Problems Worksheet Specific Heat And Calorimetry Packet Answer to calorimetry Calorimetry Packet Answers How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? How do you think you can use the calorimeter to compare the specific heat capacities of the substances listed on the. Through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and to develop a deeper. When a. Calorimetry Packet Answers.
From lessonlibraryschreiner.z21.web.core.windows.net
Specific Heat And Calorimetry Worksheet Calorimetry Packet Answers 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the molar heat of combustion. How do you think you can use the calorimeter to compare the specific heat capacities of the substances listed on the. Calorimeters can be used to find a substance’s specific heat. How much energy is needed. Calorimetry Packet Answers.
From www.studocu.com
Calorimetry Exp worksheet Experiment 9 Calorimetry With Metals Steam Method Objectives To Calorimetry Packet Answers When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. What is the relationship between heat energy and temperature? Calorimeters can be used to find a substance’s specific heat. When a substance is heated, the temperature of that substance increases. 1) if a gold ring with. Calorimetry Packet Answers.
From worksheetfullebersr.z13.web.core.windows.net
Calorimetry Worksheet Answer Key Calorimetry Packet Answers When a substance is heated, the temperature of that substance increases. Through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and to develop a deeper. What is the relationship between heat energy and temperature? How do you think you can use the calorimeter to compare the specific heat capacities of the substances listed. Calorimetry Packet Answers.
From es.scribd.com
Heat Capacity_Calorimetry Worksheet Answers Heat Capacity Heat Calorimetry Packet Answers What is the relationship between heat energy and temperature? 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in joules) has it absorbed? Through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and to develop a deeper. When a substance. Calorimetry Packet Answers.
From answerzoneschuster.z21.web.core.windows.net
Heat And Calorimetry Worksheet Answer Key Calorimetry Packet Answers How do you think you can use the calorimeter to compare the specific heat capacities of the substances listed on the. When a substance is heated, the temperature of that substance increases. What is the relationship between heat energy and temperature? How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? 1) if 0.315. Calorimetry Packet Answers.
From www.docsity.com
Calorimetry Worksheet with Answer Key Exercises Chemistry Docsity Calorimetry Packet Answers When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in joules) has it absorbed? How much energy is needed to change the temperature. Calorimetry Packet Answers.
From www.abhayjere.com
Calorimetry Worksheet Answer Key Calorimetry Packet Answers How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and to develop a deeper. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the molar heat of. Calorimetry Packet Answers.
From www.studocu.com
Calorimetry Gizmo Student Exploration Calorimetry Lab Gizmo Warmup A calorimeter is an Calorimetry Packet Answers 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in joules) has it absorbed? How do you think you can use the calorimeter to compare the specific heat capacities of the substances listed on the. Calorimeters can be used to find a substance’s specific heat. What is. Calorimetry Packet Answers.
From www.scribd.com
Activity 4 Calorimetry and Specific Heat Answer Sheet PDF Thermodynamics Calorimetry Packet Answers When a substance is heated, the temperature of that substance increases. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the molar heat of combustion. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. 1). Calorimetry Packet Answers.
From www.studocu.com
Calorimetry Examples in Chemistry Chapter 9 Studocu Calorimetry Packet Answers 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the molar heat of combustion. When a substance is heated, the temperature of that substance increases. What is the relationship between heat energy and temperature? When a hot object is placed in the calorimeter, heat energy is transferred from the object. Calorimetry Packet Answers.
From www.onlineworksheet.my.id
Calorimetry Worksheet Answer Key Onlineworksheet.my.id Calorimetry Packet Answers Calorimeters can be used to find a substance’s specific heat. What is the relationship between heat energy and temperature? Through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and to develop a deeper. When a substance is heated, the temperature of that substance increases. How do you think you can use the calorimeter. Calorimetry Packet Answers.
From www.studypool.com
SOLUTION Calorimetry lab gizmo all answers correct Studypool Calorimetry Packet Answers Calorimeters can be used to find a substance’s specific heat. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? What is the relationship between heat energy and temperature? 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in joules) has. Calorimetry Packet Answers.
From www.scribd.com
Answers TC Calorimetry Practice Questions Continuum Mechanics Physics & Mathematics Calorimetry Packet Answers When a substance is heated, the temperature of that substance increases. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the molar heat of combustion. What. Calorimetry Packet Answers.
From tomdunnacademy.org
Unlock the Answers Calorimetry Worksheet Answer Key Revealed Calorimetry Packet Answers Through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and to develop a deeper. When a substance is heated, the temperature of that substance increases. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the molar heat of combustion. Calorimeters can be used. Calorimetry Packet Answers.
From www.abhayjere.com
Calorimetry Worksheet Answer Key Calorimetry Packet Answers How do you think you can use the calorimeter to compare the specific heat capacities of the substances listed on the. 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in joules) has it absorbed? When a substance is heated, the temperature of that substance increases. 1). Calorimetry Packet Answers.
From printablezoneklaudia.z19.web.core.windows.net
Specific Heat And Calorimetry Worksheets Calorimetry Packet Answers Calorimeters can be used to find a substance’s specific heat. How do you think you can use the calorimeter to compare the specific heat capacities of the substances listed on the. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. How much energy is needed. Calorimetry Packet Answers.
From studylib.net
Calorimetry practice Calorimetry Packet Answers 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in joules) has it absorbed? Through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and to develop a deeper. How do you think you can use the calorimeter to compare the. Calorimetry Packet Answers.
From www.studocu.com
Calorimetry Lab SE All answers Name ______________________________________ Date Studocu Calorimetry Packet Answers 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in joules) has it absorbed? When a substance is heated, the temperature of that substance increases. How do you think you can use the calorimeter to compare the specific heat capacities of the substances listed on the. Through. Calorimetry Packet Answers.
From www.academia.edu
(PDF) answer key for calorimetry pogil packet mardian bocae Academia.edu Calorimetry Packet Answers What is the relationship between heat energy and temperature? When a substance is heated, the temperature of that substance increases. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the molar heat of combustion. When a hot object is placed in the calorimeter, heat energy is transferred from the object. Calorimetry Packet Answers.
From quizzlibraryzimmer.z13.web.core.windows.net
Calorimetry Questions And Answers Calorimetry Packet Answers How do you think you can use the calorimeter to compare the specific heat capacities of the substances listed on the. Through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and to develop a deeper. What is the relationship between heat energy and temperature? 1) if a gold ring with a mass of. Calorimetry Packet Answers.
From afashionlista.blogspot.com
Answer Key Calorimetry Lab Gizmo Answers Activity C / Explore Learning Circuits Gizmo Activity C Calorimetry Packet Answers 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in joules) has it absorbed? Calorimeters can be used to find a substance’s specific heat. How do you think you can use the calorimeter to compare the specific heat capacities of the substances listed on the. What is. Calorimetry Packet Answers.
From studylib.net
Calorimetry Worksheet Calorimetry Packet Answers How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? When a substance is heated, the temperature of that substance increases. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. Calorimeters can be used to find a substance’s specific. Calorimetry Packet Answers.
From printablefullombus.z21.web.core.windows.net
Calorimetry Problems Worksheets Answers Calorimetry Packet Answers How do you think you can use the calorimeter to compare the specific heat capacities of the substances listed on the. When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. What is the relationship between heat energy and temperature? When a substance is heated, the. Calorimetry Packet Answers.
From studyschoolburman.z21.web.core.windows.net
Heat And Calorimetry Worksheet Answer Key Calorimetry Packet Answers What is the relationship between heat energy and temperature? Calorimeters can be used to find a substance’s specific heat. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the molar heat of combustion. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? How. Calorimetry Packet Answers.
From www.onlineworksheet.my.id
Calorimetry Worksheet Answer Key Onlineworksheet.my.id Calorimetry Packet Answers When a substance is heated, the temperature of that substance increases. 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the molar heat of combustion. Through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and to develop a deeper. Calorimeters can be used. Calorimetry Packet Answers.
From www.studocu.com
No 9 Calorimetry w answers 32t345222 Studocu Calorimetry Packet Answers What is the relationship between heat energy and temperature? 1) if 0.315 moles of hexane (c6h14) is combusted in a bomb calorimeter containing 5.65 liters of water, calculate the molar heat of combustion. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Through these exercises, you will have the opportunity to apply the. Calorimetry Packet Answers.
From www.studocu.com
Lab 04 lab answers Exp 1 mass of cold water in the calorimeter 45 mL initial temperature of Calorimetry Packet Answers What is the relationship between heat energy and temperature? Through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and to develop a deeper. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? How do you think you can use the calorimeter to compare the specific heat. Calorimetry Packet Answers.
From www.studypool.com
SOLUTION Calorimetry lab gizmo all answers correct Studypool Calorimetry Packet Answers Through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and to develop a deeper. When a substance is heated, the temperature of that substance increases. How do you think you can use the calorimeter to compare the specific heat capacities of the substances listed on the. How much energy is needed to change. Calorimetry Packet Answers.
From studyfinder.org
How to Use a Calorimetry Gizmo to Get the Answers You Need Calorimetry Packet Answers How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? Through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and to develop a deeper. How do you think you can use the calorimeter to compare the specific heat capacities of the substances listed on the. What is. Calorimetry Packet Answers.
From www.studocu.com
Experiment 25 Calorimetry Prelaboratory Assignment CHEM 005 Studocu Calorimetry Packet Answers How do you think you can use the calorimeter to compare the specific heat capacities of the substances listed on the. How much energy is needed to change the temperature of 50.0 g of water by 15.0oc? When a substance is heated, the temperature of that substance increases. What is the relationship between heat energy and temperature? When a hot. Calorimetry Packet Answers.
From afashionlista.blogspot.com
Answer Key Calorimetry Lab Gizmo Answers Activity C / Explore Learning Circuits Gizmo Activity C Calorimetry Packet Answers When a hot object is placed in the calorimeter, heat energy is transferred from the object to the water and the water heats up. Calorimeters can be used to find a substance’s specific heat. How do you think you can use the calorimeter to compare the specific heat capacities of the substances listed on the. How much energy is needed. Calorimetry Packet Answers.
From www.studocu.com
Copy of Optional Gizmo Calorimetry Student Exploration Calorimetry Lab Directions Follow the Calorimetry Packet Answers 1) if a gold ring with a mass of 5.5 g changes temperature from 25.0 c to 28.0 c, how much energy (in joules) has it absorbed? What is the relationship between heat energy and temperature? How do you think you can use the calorimeter to compare the specific heat capacities of the substances listed on the. When a hot. Calorimetry Packet Answers.
From www.studocu.com
Gizmo Calorimetry Lab worksheet Name ______________________________________ Date Studocu Calorimetry Packet Answers Calorimeters can be used to find a substance’s specific heat. Through these exercises, you will have the opportunity to apply the theoretical concepts you have learned and to develop a deeper. When a substance is heated, the temperature of that substance increases. What is the relationship between heat energy and temperature? 1) if a gold ring with a mass of. Calorimetry Packet Answers.