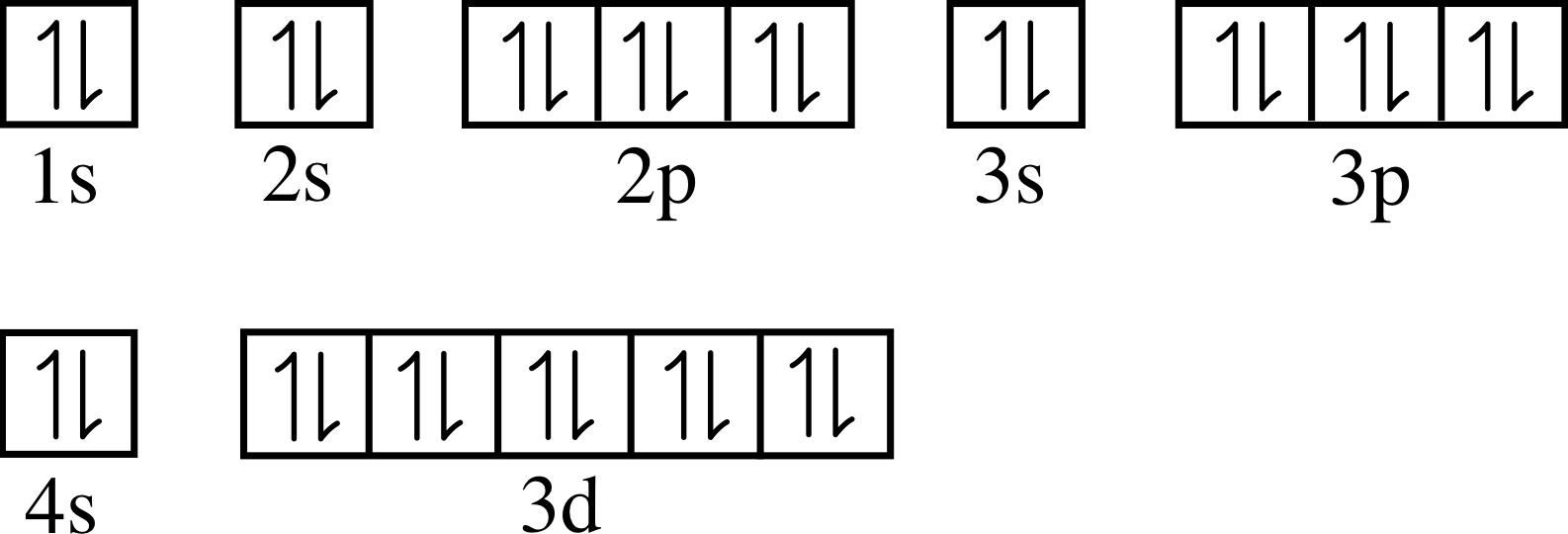
Does Zinc Take Electrons . zinc's electrical capabilities also extend to the most popular batteries. When it comes to the. zinc will give up electrons, but it must go into solution. A traditional dry cell has an outer zinc casing acting as the. in a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. The salt bridge prevents the copper ions. zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. the copper ion close to zinc will show as a positive charge locally and attract the electron. It has an atomic weight of 65.38 and. Without a salt bridge, the solution get positively charged and. zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. It is a moderately reactive metal and strong reducing agent.
from animalia-life.club
It is a moderately reactive metal and strong reducing agent. zinc's electrical capabilities also extend to the most popular batteries. in a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. It has an atomic weight of 65.38 and. zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. The salt bridge prevents the copper ions. When it comes to the. zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. zinc will give up electrons, but it must go into solution. Without a salt bridge, the solution get positively charged and.
Zinc Electron Configuration
Does Zinc Take Electrons zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. The salt bridge prevents the copper ions. zinc will give up electrons, but it must go into solution. the copper ion close to zinc will show as a positive charge locally and attract the electron. A traditional dry cell has an outer zinc casing acting as the. zinc's electrical capabilities also extend to the most popular batteries. It is a moderately reactive metal and strong reducing agent. in a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. When it comes to the. Without a salt bridge, the solution get positively charged and. It has an atomic weight of 65.38 and. zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30.
From www.pinterest.com
22 best Lessons to learn images on Pinterest School projects, Atomic Does Zinc Take Electrons the copper ion close to zinc will show as a positive charge locally and attract the electron. Without a salt bridge, the solution get positively charged and. zinc will give up electrons, but it must go into solution. zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of. Does Zinc Take Electrons.
From animalia-life.club
Zinc Electron Configuration Does Zinc Take Electrons It is a moderately reactive metal and strong reducing agent. When it comes to the. zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. the copper ion close to zinc will show as a positive charge locally and attract the electron. in a spontaneous reaction electrons. Does Zinc Take Electrons.
From animalia-life.club
Zinc Electron Configuration Does Zinc Take Electrons A traditional dry cell has an outer zinc casing acting as the. the copper ion close to zinc will show as a positive charge locally and attract the electron. zinc will give up electrons, but it must go into solution. It is a moderately reactive metal and strong reducing agent. The salt bridge prevents the copper ions. When. Does Zinc Take Electrons.
From socratic.org
Can you describe the process that releases electrons in a zinc copper Does Zinc Take Electrons the copper ion close to zinc will show as a positive charge locally and attract the electron. It is a moderately reactive metal and strong reducing agent. A traditional dry cell has an outer zinc casing acting as the. in a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the. Does Zinc Take Electrons.
From www.dreamstime.com
Zinc stock illustration. Illustration of render, chemistry 139650988 Does Zinc Take Electrons When it comes to the. zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. It has an atomic weight of 65.38 and. Without a salt bridge, the solution get positively charged and. zinc's electrical capabilities also extend to the most popular batteries. The salt bridge. Does Zinc Take Electrons.
From chemistry.stackexchange.com
How to get the condensed electron configuration for Zinc ion Does Zinc Take Electrons zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. in a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. A traditional dry cell has an outer zinc casing acting as the. Without a salt. Does Zinc Take Electrons.
From courses.lumenlearning.com
Galvanic Cells General Chemistry Does Zinc Take Electrons A traditional dry cell has an outer zinc casing acting as the. zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. in a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. the copper. Does Zinc Take Electrons.
From www.slideserve.com
PPT PS4 The Atom & the Periodic Table PowerPoint Presentation ID Does Zinc Take Electrons The salt bridge prevents the copper ions. zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. A traditional dry cell has an outer zinc casing acting as the. in a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up. Does Zinc Take Electrons.
From elchoroukhost.net
Periodic Table Zinc Protons Neutrons Electrons Elcho Table Does Zinc Take Electrons zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. Without a salt bridge, the solution get positively charged and. A traditional dry cell has an outer zinc casing acting as the. When it comes to the. zinc will give up electrons, but it must go. Does Zinc Take Electrons.
From dxonyecim.blob.core.windows.net
Zn Electron Configuration Diagram at Beatrice Fitch blog Does Zinc Take Electrons zinc will give up electrons, but it must go into solution. Without a salt bridge, the solution get positively charged and. in a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. It has an atomic weight of 65.38 and. zinc is the 30th element in the. Does Zinc Take Electrons.
From www.youtube.com
How to Find the Valence Electrons for Zinc (Zn) YouTube Does Zinc Take Electrons It has an atomic weight of 65.38 and. zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. the copper ion close to zinc will show as a positive charge locally and attract the electron. zinc's electrical capabilities also extend to the most popular batteries.. Does Zinc Take Electrons.
From schoolbag.info
Figure 12.1. Daniell Cell In this galvanic cell, zinc is the anode and Does Zinc Take Electrons zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. zinc will give up electrons, but it must go into solution. zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. A traditional dry cell. Does Zinc Take Electrons.
From www.youtube.com
How many valence electrons are in zinc? YouTube Does Zinc Take Electrons It has an atomic weight of 65.38 and. in a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. It is a moderately reactive metal and strong reducing agent. zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of. Does Zinc Take Electrons.
From animalia-life.club
Zinc Electron Configuration Does Zinc Take Electrons zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. A traditional dry cell has an outer zinc casing acting as the. zinc's electrical capabilities also extend to the most popular batteries. It has an atomic weight of 65.38 and. in a spontaneous reaction electrons. Does Zinc Take Electrons.
From www.pinterest.com.au
What Happens When Atoms Bond infographic diagram showing how electrons Does Zinc Take Electrons zinc's electrical capabilities also extend to the most popular batteries. When it comes to the. the copper ion close to zinc will show as a positive charge locally and attract the electron. It has an atomic weight of 65.38 and. Without a salt bridge, the solution get positively charged and. It is a moderately reactive metal and strong. Does Zinc Take Electrons.
From material-properties.org
Zinc Periodic Table and Atomic Properties Does Zinc Take Electrons zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. zinc will give up electrons, but it must go into solution. the copper ion close to zinc will show as a positive charge locally and attract the electron. The salt bridge prevents the copper ions.. Does Zinc Take Electrons.
From www.chegg.com
Solved The metal zinc provides two conduction electrons per Does Zinc Take Electrons in a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. When it comes to the. zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. zinc has an electron configuration of [ar]3d 10 4s 2 and. Does Zinc Take Electrons.
From www.youtube.com
How to find Protons & Electrons for the Zn 2+ (Zinc ion) YouTube Does Zinc Take Electrons It has an atomic weight of 65.38 and. zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. The salt bridge prevents the copper ions. zinc will give up electrons, but it must go into solution. It is a moderately reactive metal and strong reducing agent.. Does Zinc Take Electrons.
From material-properties.org
Zinc Protons Neutrons Electrons Electron Configuration Does Zinc Take Electrons It has an atomic weight of 65.38 and. When it comes to the. zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. zinc's electrical capabilities also extend to the most popular batteries. A traditional dry cell has an outer zinc casing acting as the. in a. Does Zinc Take Electrons.
From blog.thepipingmart.com
Difference Between Copper and Zinc Does Zinc Take Electrons zinc will give up electrons, but it must go into solution. Without a salt bridge, the solution get positively charged and. zinc's electrical capabilities also extend to the most popular batteries. It has an atomic weight of 65.38 and. It is a moderately reactive metal and strong reducing agent. When it comes to the. the copper ion. Does Zinc Take Electrons.
From www.sciencephoto.com
Zinc, atomic structure Stock Image C013/1553 Science Photo Library Does Zinc Take Electrons Without a salt bridge, the solution get positively charged and. in a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. A traditional dry cell has an outer zinc casing acting as the. zinc will give up electrons, but it must go into solution. zinc has an. Does Zinc Take Electrons.
From www.researchgate.net
Electrochemical reversible cell containing silver and zinc in Does Zinc Take Electrons zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. The salt bridge prevents the copper ions. zinc will give up electrons, but it must go into solution. zinc's electrical capabilities also extend to the most popular batteries. the copper ion close to zinc will show. Does Zinc Take Electrons.
From www.alamy.com
Zn Zinc, Periodic Table of the Elements, Shell Structure of Zinc Does Zinc Take Electrons zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. It is a moderately reactive metal and strong reducing agent. Without a salt bridge, the solution get positively charged and. It has an atomic weight of 65.38 and. The salt bridge prevents the copper ions. the copper ion. Does Zinc Take Electrons.
From www.numerade.com
SOLVED Write the complete electron configuration for the zinc atom Does Zinc Take Electrons The salt bridge prevents the copper ions. Without a salt bridge, the solution get positively charged and. zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. zinc's electrical capabilities also extend to the most popular batteries. When it comes to the. in a spontaneous. Does Zinc Take Electrons.
From www.sciencephoto.com
Zinc electron configuration Stock Image C029/5029 Science Photo Does Zinc Take Electrons zinc's electrical capabilities also extend to the most popular batteries. The salt bridge prevents the copper ions. It has an atomic weight of 65.38 and. When it comes to the. It is a moderately reactive metal and strong reducing agent. zinc will give up electrons, but it must go into solution. zinc is the 30th element in. Does Zinc Take Electrons.
From dwsestamparia.com.br
Zinc Health Benefits Food And Supplements You Need To Know, 48 OFF Does Zinc Take Electrons It is a moderately reactive metal and strong reducing agent. Without a salt bridge, the solution get positively charged and. The salt bridge prevents the copper ions. zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. in a spontaneous reaction electrons leave the zinc, go. Does Zinc Take Electrons.
From animalia-life.club
Zinc Electron Configuration Does Zinc Take Electrons zinc will give up electrons, but it must go into solution. It has an atomic weight of 65.38 and. When it comes to the. zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. the copper ion close to zinc will show as a positive. Does Zinc Take Electrons.
From valenceelectrons.com
Complete Electron Configuration for Zinc (Zn, Zn2+ ion) Does Zinc Take Electrons Without a salt bridge, the solution get positively charged and. zinc's electrical capabilities also extend to the most popular batteries. When it comes to the. zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. the copper ion close to zinc will show as a. Does Zinc Take Electrons.
From valenceelectrons.com
How to Find the Valence Electrons for Carbon(C)? Does Zinc Take Electrons The salt bridge prevents the copper ions. When it comes to the. zinc will give up electrons, but it must go into solution. the copper ion close to zinc will show as a positive charge locally and attract the electron. zinc's electrical capabilities also extend to the most popular batteries. It is a moderately reactive metal and. Does Zinc Take Electrons.
From courses.lumenlearning.com
6.1 Lewis Electron Dot Symbols Introductory Chemistry Does Zinc Take Electrons It is a moderately reactive metal and strong reducing agent. zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. It has an atomic weight of 65.38 and. A traditional dry cell has an outer zinc casing acting as the. When it comes to the. zinc has an. Does Zinc Take Electrons.
From chem.libretexts.org
Chapter 19.7 Electrolysis Chemistry LibreTexts Does Zinc Take Electrons the copper ion close to zinc will show as a positive charge locally and attract the electron. in a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. It is a moderately reactive metal and strong reducing agent. Without a salt bridge, the solution get positively charged and.. Does Zinc Take Electrons.
From exowsidwq.blob.core.windows.net
Zinc How To Find Charge at Patel blog Does Zinc Take Electrons Without a salt bridge, the solution get positively charged and. zinc has an electron configuration of [ar]3d 10 4s 2 and is a member of the group 12 of the periodic table. zinc is the 30th element in the periodic table and has a symbol of zn and atomic number of 30. A traditional dry cell has an. Does Zinc Take Electrons.
From blog.thepipingmart.com
Zinc and Copper Redox Reaction Equation Does Zinc Take Electrons the copper ion close to zinc will show as a positive charge locally and attract the electron. It is a moderately reactive metal and strong reducing agent. zinc's electrical capabilities also extend to the most popular batteries. A traditional dry cell has an outer zinc casing acting as the. When it comes to the. in a spontaneous. Does Zinc Take Electrons.
From www.youtube.com
How to find the Number of Protons, Electrons, Neutrons for Zinc (Zn Does Zinc Take Electrons the copper ion close to zinc will show as a positive charge locally and attract the electron. zinc will give up electrons, but it must go into solution. in a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the copper ions. It is a moderately reactive metal and strong. Does Zinc Take Electrons.
From golace.blogspot.com
zinc orbital diagram Golace Does Zinc Take Electrons Without a salt bridge, the solution get positively charged and. the copper ion close to zinc will show as a positive charge locally and attract the electron. zinc will give up electrons, but it must go into solution. in a spontaneous reaction electrons leave the zinc, go through the wire and are then taken up by the. Does Zinc Take Electrons.