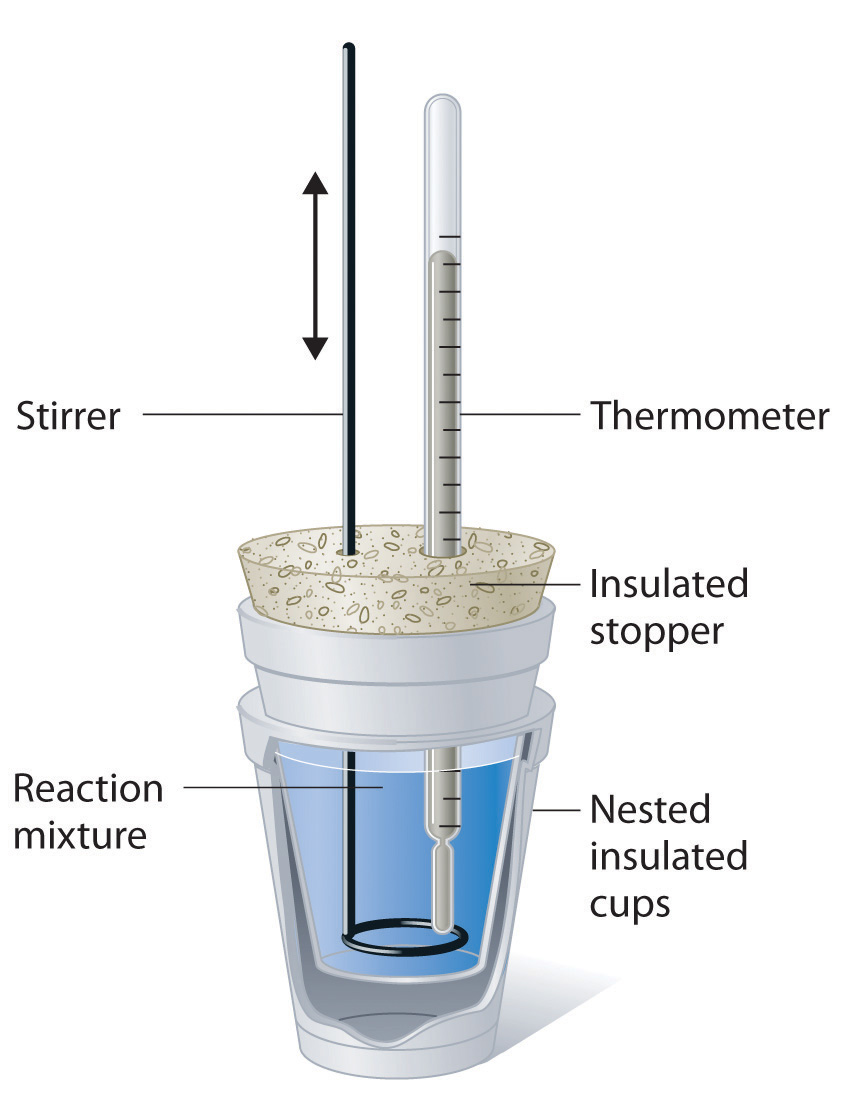
What Is Calorimeter Example . calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. Most students likely do not remember using such a. a calorimeter is a device used to measure the quantity of heat transferred to or from an object. It uses devices called calorimeters, which measure the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. apply the first law of thermodynamics to calorimetry. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
from saylordotorg.github.io
Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Most students likely do not remember using such a. apply the first law of thermodynamics to calorimetry. a calorimeter is a device used to measure the quantity of heat transferred to or from an object. It uses devices called calorimeters, which measure the.
Calorimetry
What Is Calorimeter Example a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. apply the first law of thermodynamics to calorimetry. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. Most students likely do not remember using such a. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It uses devices called calorimeters, which measure the. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device used to measure the quantity of heat transferred to or from an object.
From www.youtube.com
CALORIMETRY EXAMPLE 3 YouTube What Is Calorimeter Example Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Most students likely do not remember using such a. It uses devices called calorimeters, which measure the. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the set of techniques. What Is Calorimeter Example.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID3850751 What Is Calorimeter Example Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. apply the first law of thermodynamics to calorimetry. Most students likely do not remember using such a. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimeter, device for measuring the. What Is Calorimeter Example.
From www.scienceabc.com
Molar Heat Capacity Definition, Formula, Equation, Calculation What Is Calorimeter Example apply the first law of thermodynamics to calorimetry. Most students likely do not remember using such a. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. Compare heat flow from hot to cold. What Is Calorimeter Example.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 What Is Calorimeter Example a calorimeter is a device used to measure the quantity of heat transferred to or from an object. Most students likely do not remember using such a. It uses devices called calorimeters, which measure the. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. calorimeter, device for measuring the heat developed during. What Is Calorimeter Example.
From saylordotorg.github.io
Calorimetry What Is Calorimeter Example It uses devices called calorimeters, which measure the. a calorimeter is a device used to measure the quantity of heat transferred to or from an object. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating. What Is Calorimeter Example.
From www.laboratory-equipment.com
Calorimeter Features, Styles, Types, and Differences What Is Calorimeter Example It uses devices called calorimeters, which measure the. apply the first law of thermodynamics to calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. calorimetry is the set of techniques. What Is Calorimeter Example.
From www.youtube.com
Principle of Calorimetry YouTube What Is Calorimeter Example Most students likely do not remember using such a. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes.. What Is Calorimeter Example.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6912350 What Is Calorimeter Example It uses devices called calorimeters, which measure the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device used to measure the quantity of heat transferred to or from an object. apply the first law of thermodynamics to calorimetry. Most students likely do not remember using such. What Is Calorimeter Example.
From www.youtube.com
Physics 9.09b Calorimetry Example 1 YouTube What Is Calorimeter Example calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. a calorimeter is a device used to measure the quantity of heat transferred to or from an object. It uses devices called calorimeters, which measure the. calorimetry is the set of techniques used to measure enthalpy changes during. What Is Calorimeter Example.
From study.com
Calorimetry Definition, Equation & Types Lesson What Is Calorimeter Example calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. a calorimeter is a device used to measure the quantity of heat transferred to or from an object. Most students likely do not remember using such a. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. What Is Calorimeter Example.
From www.tessshebaylo.com
Equation For Calorimetry Tessshebaylo What Is Calorimeter Example a calorimeter is a device used to measure the quantity of heat transferred to or from an object. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. apply the first law of thermodynamics to calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical. What Is Calorimeter Example.
From saylordotorg.github.io
Calorimetry What Is Calorimeter Example It uses devices called calorimeters, which measure the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. apply the first law of thermodynamics to calorimetry. calorimeter, device for measuring the heat. What Is Calorimeter Example.
From www.thoughtco.com
Calorimeter Definition in Chemistry What Is Calorimeter Example a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. It uses devices called calorimeters, which measure the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. . What Is Calorimeter Example.
From exotbfkyp.blob.core.windows.net
What Is Calorimetry And Who Would Be Interested In Performing It at What Is Calorimeter Example a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Most students likely do not remember using such a. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. calorimetry is the set of techniques used to measure enthalpy. What Is Calorimeter Example.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry What Is Calorimeter Example a calorimeter is a device used to measure the quantity of heat transferred to or from an object. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. Most students likely do not remember. What Is Calorimeter Example.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube What Is Calorimeter Example Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device used to measure the quantity of heat transferred to or from an object. It uses devices called calorimeters, which measure the. Most students likely do not remember using such a. calorimetry is the set of techniques used. What Is Calorimeter Example.
From www.laboratory-equipment.com
Laboratory Calorimeters from IKA What Is Calorimeter Example Most students likely do not remember using such a. apply the first law of thermodynamics to calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. It uses. What Is Calorimeter Example.
From study.com
Bomb Calorimeter Uses, Equations & Examples Lesson What Is Calorimeter Example a calorimeter is a device used to measure the quantity of heat transferred to or from an object. Most students likely do not remember using such a. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. a calorimeter is a device used to measure the amount of. What Is Calorimeter Example.
From glossary.periodni.com
Bomb calorimeter Chemistry Dictionary & Glossary What Is Calorimeter Example It uses devices called calorimeters, which measure the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. . What Is Calorimeter Example.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii What Is Calorimeter Example a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. apply the first law of thermodynamics to calorimetry. a calorimeter is a device used to measure the quantity of heat transferred to or from an object. It uses devices called calorimeters, which measure the. Compare heat flow from. What Is Calorimeter Example.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts What Is Calorimeter Example a calorimeter is a device used to measure the quantity of heat transferred to or from an object. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It uses devices called calorimeters, which measure the. Most students likely do not remember using such a. calorimeter, device for. What Is Calorimeter Example.
From www.edrawmax.com
Calorimetry Lab Report EdrawMax Template What Is Calorimeter Example Most students likely do not remember using such a. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. It uses devices called calorimeters, which measure the. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. apply the. What Is Calorimeter Example.
From www.studypool.com
SOLUTION Bomb calorimeter explain with diagram and example? Studypool What Is Calorimeter Example a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It uses devices called calorimeters, which measure the. a calorimeter is a device used to measure the quantity of heat transferred to or from an object. Most students likely do not remember using such a. calorimeter, device for. What Is Calorimeter Example.
From www.youtube.com
How to find calorimeter constant YouTube What Is Calorimeter Example calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. It uses devices called calorimeters, which measure the. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. apply the first law of thermodynamics to calorimetry. Compare heat flow. What Is Calorimeter Example.
From www.youtube.com
050 Calorimetry YouTube What Is Calorimeter Example calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. It uses devices called. What Is Calorimeter Example.
From dxodontbm.blob.core.windows.net
What's Calorimetry In Physics at James Powell blog What Is Calorimeter Example Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Most students likely do not remember using such a. It uses devices called calorimeters, which measure the. a calorimeter is a device used to measure the quantity of heat transferred to or from an object. apply the first law of thermodynamics to. What Is Calorimeter Example.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica What Is Calorimeter Example a calorimeter is a device used to measure the quantity of heat transferred to or from an object. Most students likely do not remember using such a. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects. What Is Calorimeter Example.
From physique.ensc-rennes.fr
TP calorimetry What Is Calorimeter Example a calorimeter is a device used to measure the quantity of heat transferred to or from an object. calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. a calorimeter is a device. What Is Calorimeter Example.
From www.animalia-life.club
Calorimeter Diagram What Is Calorimeter Example apply the first law of thermodynamics to calorimetry. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Compare heat flow from hot to cold objects in an ideal. What Is Calorimeter Example.
From www.youtube.com
Physics 9.09g Calorimetry Example 2 YouTube What Is Calorimeter Example It uses devices called calorimeters, which measure the. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. a calorimeter is a device used to measure the amount of heat involved in a. What Is Calorimeter Example.
From thechemistrynotes.com
Calorimeter Definition, Types and Uses What Is Calorimeter Example calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for calculating the heat. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. apply the first law of thermodynamics to calorimetry.. What Is Calorimeter Example.
From www.pinterest.com.au
Pin on Science Friday What Is Calorimeter Example Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. It uses devices called calorimeters, which measure the. apply the first law of thermodynamics to calorimetry. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. a calorimeter is a device used. What Is Calorimeter Example.
From studylib.net
Calorimetry What Is Calorimeter Example Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. Most students likely do not remember using such a. It uses devices called calorimeters, which measure the. a calorimeter is a device used to measure the quantity of heat transferred to or from an object. calorimeter, device for measuring the heat developed. What Is Calorimeter Example.
From thermonine92.blogspot.com
Thermochemistry Calorimeter What Is Calorimeter Example calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. a calorimeter is a device used to measure the quantity of heat transferred to or from an object. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Most students likely do not remember. What Is Calorimeter Example.
From materialschreiner.z19.web.core.windows.net
Calorimetry Sample Problems With Solutions What Is Calorimeter Example apply the first law of thermodynamics to calorimetry. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real calorimeter. It uses devices called calorimeters, which measure the. a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the set of techniques. What Is Calorimeter Example.