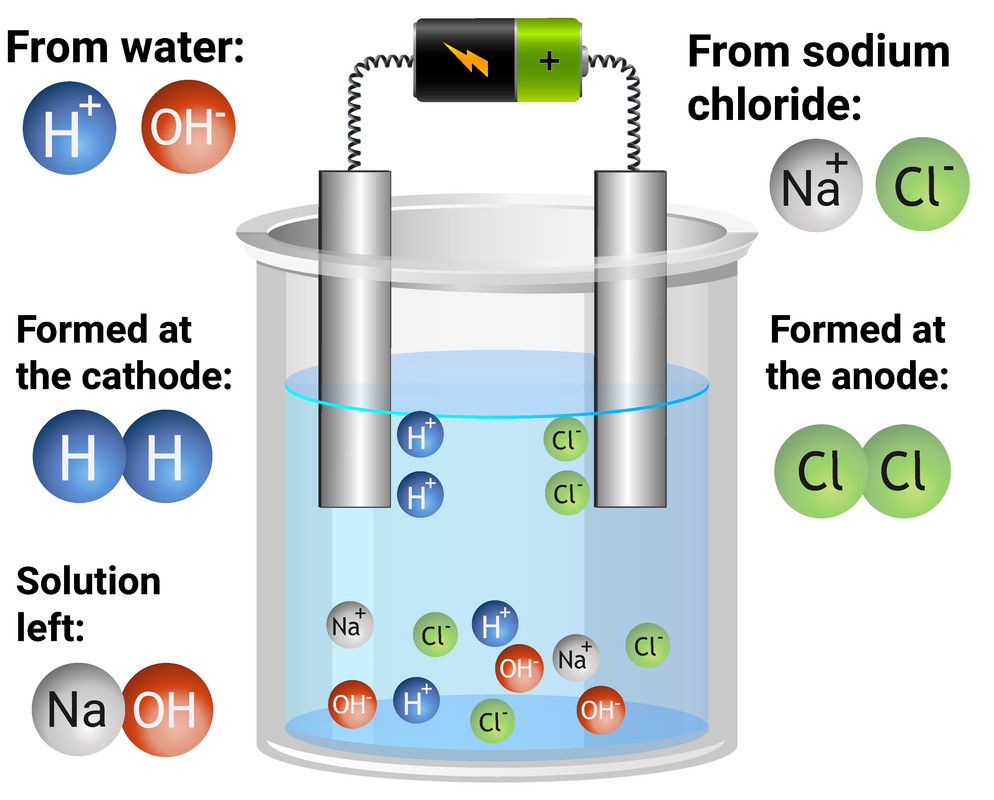
What Happens At The Cathode And Anode During Electrolysis . This occurs as copper atoms are oxidised at the anode and form ions while copper ions are reduced at the. The table summarises the product formed at the anode during the electrolysis of different electrolytes close electrolyte a substance. Either the metal is deposited or you get hydrogen. What happens at the cathode? Positive ions, cations close cation an atom or group of atoms that have lost electrons and become positively charged. The positive anode attracts anions toward it, while the negative. The power source pumps the new electrons on the anode along the circuit to replace the ones taken off the cathode. At the negative electrode (cathode) a reduction reaction occurs. The cathode increases in mass while the anode decreases. The battery pumps electrons away from the anode (making it positive) and into the cathode (making it negative). Positive ions are attracted to the cathode, where they pick up one or more electrons and are discharged. When a steady current is passed through the solution, the net result is that silver metal is removed from the anode and deposited on the cathode.
from www.revisechemistry.uk
Either the metal is deposited or you get hydrogen. At the negative electrode (cathode) a reduction reaction occurs. The battery pumps electrons away from the anode (making it positive) and into the cathode (making it negative). This occurs as copper atoms are oxidised at the anode and form ions while copper ions are reduced at the. The power source pumps the new electrons on the anode along the circuit to replace the ones taken off the cathode. When a steady current is passed through the solution, the net result is that silver metal is removed from the anode and deposited on the cathode. Positive ions are attracted to the cathode, where they pick up one or more electrons and are discharged. The table summarises the product formed at the anode during the electrolysis of different electrolytes close electrolyte a substance. What happens at the cathode? The cathode increases in mass while the anode decreases.
Electrolysis OCR Gateway C3 revisechemistry.uk
What Happens At The Cathode And Anode During Electrolysis Either the metal is deposited or you get hydrogen. Positive ions, cations close cation an atom or group of atoms that have lost electrons and become positively charged. The positive anode attracts anions toward it, while the negative. At the negative electrode (cathode) a reduction reaction occurs. What happens at the cathode? The table summarises the product formed at the anode during the electrolysis of different electrolytes close electrolyte a substance. Either the metal is deposited or you get hydrogen. The power source pumps the new electrons on the anode along the circuit to replace the ones taken off the cathode. This occurs as copper atoms are oxidised at the anode and form ions while copper ions are reduced at the. The battery pumps electrons away from the anode (making it positive) and into the cathode (making it negative). When a steady current is passed through the solution, the net result is that silver metal is removed from the anode and deposited on the cathode. The cathode increases in mass while the anode decreases. Positive ions are attracted to the cathode, where they pick up one or more electrons and are discharged.
From www.slideserve.com
PPT Electrolysis L.O. I know and can use the terms electrolyte What Happens At The Cathode And Anode During Electrolysis The table summarises the product formed at the anode during the electrolysis of different electrolytes close electrolyte a substance. The cathode increases in mass while the anode decreases. This occurs as copper atoms are oxidised at the anode and form ions while copper ions are reduced at the. Positive ions, cations close cation an atom or group of atoms that. What Happens At The Cathode And Anode During Electrolysis.
From hadassah-has-friedman.blogspot.com
Anode and Cathode in Electrolysis HadassahhasFriedman What Happens At The Cathode And Anode During Electrolysis When a steady current is passed through the solution, the net result is that silver metal is removed from the anode and deposited on the cathode. Positive ions, cations close cation an atom or group of atoms that have lost electrons and become positively charged. At the negative electrode (cathode) a reduction reaction occurs. Either the metal is deposited or. What Happens At The Cathode And Anode During Electrolysis.
From wisc.pb.unizin.org
D41.4 Electrolysis Chemistry 109 Fall 2021 What Happens At The Cathode And Anode During Electrolysis The power source pumps the new electrons on the anode along the circuit to replace the ones taken off the cathode. Positive ions are attracted to the cathode, where they pick up one or more electrons and are discharged. The table summarises the product formed at the anode during the electrolysis of different electrolytes close electrolyte a substance. The battery. What Happens At The Cathode And Anode During Electrolysis.
From general.chemistrysteps.com
Electrolysis of Water Chemistry Steps What Happens At The Cathode And Anode During Electrolysis The positive anode attracts anions toward it, while the negative. At the negative electrode (cathode) a reduction reaction occurs. When a steady current is passed through the solution, the net result is that silver metal is removed from the anode and deposited on the cathode. The table summarises the product formed at the anode during the electrolysis of different electrolytes. What Happens At The Cathode And Anode During Electrolysis.
From www.collegesearch.in
Cathode and Anode Definition, Examples, Differences CollegeSearch What Happens At The Cathode And Anode During Electrolysis The battery pumps electrons away from the anode (making it positive) and into the cathode (making it negative). Positive ions are attracted to the cathode, where they pick up one or more electrons and are discharged. The power source pumps the new electrons on the anode along the circuit to replace the ones taken off the cathode. This occurs as. What Happens At The Cathode And Anode During Electrolysis.
From userpartfrieda.z21.web.core.windows.net
Cathode Electrolyte Circuit Diagram What Happens At The Cathode And Anode During Electrolysis The power source pumps the new electrons on the anode along the circuit to replace the ones taken off the cathode. This occurs as copper atoms are oxidised at the anode and form ions while copper ions are reduced at the. The battery pumps electrons away from the anode (making it positive) and into the cathode (making it negative). At. What Happens At The Cathode And Anode During Electrolysis.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk What Happens At The Cathode And Anode During Electrolysis This occurs as copper atoms are oxidised at the anode and form ions while copper ions are reduced at the. What happens at the cathode? The cathode increases in mass while the anode decreases. The positive anode attracts anions toward it, while the negative. When a steady current is passed through the solution, the net result is that silver metal. What Happens At The Cathode And Anode During Electrolysis.
From www.revisechemistry.uk
Electrolysis OCR Gateway C3 revisechemistry.uk What Happens At The Cathode And Anode During Electrolysis At the negative electrode (cathode) a reduction reaction occurs. What happens at the cathode? The battery pumps electrons away from the anode (making it positive) and into the cathode (making it negative). The cathode increases in mass while the anode decreases. Positive ions, cations close cation an atom or group of atoms that have lost electrons and become positively charged.. What Happens At The Cathode And Anode During Electrolysis.
From www.thoughtco.com
How to Define Anode and Cathode What Happens At The Cathode And Anode During Electrolysis Either the metal is deposited or you get hydrogen. What happens at the cathode? The positive anode attracts anions toward it, while the negative. When a steady current is passed through the solution, the net result is that silver metal is removed from the anode and deposited on the cathode. The power source pumps the new electrons on the anode. What Happens At The Cathode And Anode During Electrolysis.
From www.youtube.com
Cathode and Anode Quick differences and comparisons YouTube What Happens At The Cathode And Anode During Electrolysis What happens at the cathode? The power source pumps the new electrons on the anode along the circuit to replace the ones taken off the cathode. Either the metal is deposited or you get hydrogen. Positive ions, cations close cation an atom or group of atoms that have lost electrons and become positively charged. At the negative electrode (cathode) a. What Happens At The Cathode And Anode During Electrolysis.
From chemistry.stackexchange.com
physical chemistry Positive or Negative Anode/Cathode in Electrolytic What Happens At The Cathode And Anode During Electrolysis When a steady current is passed through the solution, the net result is that silver metal is removed from the anode and deposited on the cathode. This occurs as copper atoms are oxidised at the anode and form ions while copper ions are reduced at the. The positive anode attracts anions toward it, while the negative. The battery pumps electrons. What Happens At The Cathode And Anode During Electrolysis.
From haydenjoyssheppard.blogspot.com
Anode and Cathode in Electrolysis HaydenjoysSheppard What Happens At The Cathode And Anode During Electrolysis Positive ions are attracted to the cathode, where they pick up one or more electrons and are discharged. The positive anode attracts anions toward it, while the negative. Either the metal is deposited or you get hydrogen. The battery pumps electrons away from the anode (making it positive) and into the cathode (making it negative). The power source pumps the. What Happens At The Cathode And Anode During Electrolysis.
From madisonmeowmercado.blogspot.com
Anode and Cathode in Electrolysis What Happens At The Cathode And Anode During Electrolysis What happens at the cathode? At the negative electrode (cathode) a reduction reaction occurs. Positive ions are attracted to the cathode, where they pick up one or more electrons and are discharged. Positive ions, cations close cation an atom or group of atoms that have lost electrons and become positively charged. The positive anode attracts anions toward it, while the. What Happens At The Cathode And Anode During Electrolysis.
From fixlibrarygedwaaldebx.z21.web.core.windows.net
Cathode Electrolyte Circuit Diagram What Happens At The Cathode And Anode During Electrolysis What happens at the cathode? The battery pumps electrons away from the anode (making it positive) and into the cathode (making it negative). The power source pumps the new electrons on the anode along the circuit to replace the ones taken off the cathode. The positive anode attracts anions toward it, while the negative. Either the metal is deposited or. What Happens At The Cathode And Anode During Electrolysis.
From www.vedantu.com
Cathode and Anode Definition and Difference Between Anode and Cathode What Happens At The Cathode And Anode During Electrolysis At the negative electrode (cathode) a reduction reaction occurs. The positive anode attracts anions toward it, while the negative. The table summarises the product formed at the anode during the electrolysis of different electrolytes close electrolyte a substance. Either the metal is deposited or you get hydrogen. When a steady current is passed through the solution, the net result is. What Happens At The Cathode And Anode During Electrolysis.
From ceodjbtu.blob.core.windows.net
Water Electrolysis Engine at Heidi Rivera blog What Happens At The Cathode And Anode During Electrolysis When a steady current is passed through the solution, the net result is that silver metal is removed from the anode and deposited on the cathode. At the negative electrode (cathode) a reduction reaction occurs. Either the metal is deposited or you get hydrogen. Positive ions are attracted to the cathode, where they pick up one or more electrons and. What Happens At The Cathode And Anode During Electrolysis.
From mungfali.com
Electrolysis Process Diagram What Happens At The Cathode And Anode During Electrolysis Positive ions are attracted to the cathode, where they pick up one or more electrons and are discharged. The table summarises the product formed at the anode during the electrolysis of different electrolytes close electrolyte a substance. This occurs as copper atoms are oxidised at the anode and form ions while copper ions are reduced at the. The battery pumps. What Happens At The Cathode And Anode During Electrolysis.
From www.slideserve.com
PPT Electrolysis L.O. I know and can use the terms electrolyte What Happens At The Cathode And Anode During Electrolysis This occurs as copper atoms are oxidised at the anode and form ions while copper ions are reduced at the. At the negative electrode (cathode) a reduction reaction occurs. Either the metal is deposited or you get hydrogen. The positive anode attracts anions toward it, while the negative. The cathode increases in mass while the anode decreases. Positive ions are. What Happens At The Cathode And Anode During Electrolysis.
From www.researchgate.net
14 The electrolysis of water produces oxygen at the anode and hydrogen What Happens At The Cathode And Anode During Electrolysis This occurs as copper atoms are oxidised at the anode and form ions while copper ions are reduced at the. At the negative electrode (cathode) a reduction reaction occurs. The battery pumps electrons away from the anode (making it positive) and into the cathode (making it negative). The table summarises the product formed at the anode during the electrolysis of. What Happens At The Cathode And Anode During Electrolysis.
From study.com
Electrolysis of Aqueous Solutions Lesson What Happens At The Cathode And Anode During Electrolysis Positive ions are attracted to the cathode, where they pick up one or more electrons and are discharged. The cathode increases in mass while the anode decreases. This occurs as copper atoms are oxidised at the anode and form ions while copper ions are reduced at the. Positive ions, cations close cation an atom or group of atoms that have. What Happens At The Cathode And Anode During Electrolysis.
From partdiagramvovneyve.z21.web.core.windows.net
Cathode Charge In Electrolysis What Happens At The Cathode And Anode During Electrolysis Positive ions, cations close cation an atom or group of atoms that have lost electrons and become positively charged. What happens at the cathode? The cathode increases in mass while the anode decreases. Positive ions are attracted to the cathode, where they pick up one or more electrons and are discharged. The battery pumps electrons away from the anode (making. What Happens At The Cathode And Anode During Electrolysis.
From www.slideserve.com
PPT Topic Electrochemical Cells PowerPoint Presentation, free What Happens At The Cathode And Anode During Electrolysis When a steady current is passed through the solution, the net result is that silver metal is removed from the anode and deposited on the cathode. The positive anode attracts anions toward it, while the negative. Either the metal is deposited or you get hydrogen. Positive ions are attracted to the cathode, where they pick up one or more electrons. What Happens At The Cathode And Anode During Electrolysis.
From socratic.org
What happens on the cathode during the electrolysis of a copper(II What Happens At The Cathode And Anode During Electrolysis The cathode increases in mass while the anode decreases. The positive anode attracts anions toward it, while the negative. Positive ions are attracted to the cathode, where they pick up one or more electrons and are discharged. The power source pumps the new electrons on the anode along the circuit to replace the ones taken off the cathode. The table. What Happens At The Cathode And Anode During Electrolysis.
From byjus.com
8. During electrolysis of water,what are the charges carried by anode What Happens At The Cathode And Anode During Electrolysis The power source pumps the new electrons on the anode along the circuit to replace the ones taken off the cathode. Positive ions, cations close cation an atom or group of atoms that have lost electrons and become positively charged. What happens at the cathode? The table summarises the product formed at the anode during the electrolysis of different electrolytes. What Happens At The Cathode And Anode During Electrolysis.
From www.teachoo.com
During the electrolytic refining of copper what happens at the anode? What Happens At The Cathode And Anode During Electrolysis The power source pumps the new electrons on the anode along the circuit to replace the ones taken off the cathode. When a steady current is passed through the solution, the net result is that silver metal is removed from the anode and deposited on the cathode. This occurs as copper atoms are oxidised at the anode and form ions. What Happens At The Cathode And Anode During Electrolysis.
From exoookubp.blob.core.windows.net
What Are The Anode And Cathode at Ada Smith blog What Happens At The Cathode And Anode During Electrolysis This occurs as copper atoms are oxidised at the anode and form ions while copper ions are reduced at the. The positive anode attracts anions toward it, while the negative. The cathode increases in mass while the anode decreases. Positive ions are attracted to the cathode, where they pick up one or more electrons and are discharged. The battery pumps. What Happens At The Cathode And Anode During Electrolysis.
From circuitlibscombrid.z13.web.core.windows.net
What Happens At The Cathode In Electrolysis What Happens At The Cathode And Anode During Electrolysis Positive ions, cations close cation an atom or group of atoms that have lost electrons and become positively charged. The table summarises the product formed at the anode during the electrolysis of different electrolytes close electrolyte a substance. When a steady current is passed through the solution, the net result is that silver metal is removed from the anode and. What Happens At The Cathode And Anode During Electrolysis.
From guidediagramschoolboys.z21.web.core.windows.net
What Forms At The Cathode During Electrolysis What Happens At The Cathode And Anode During Electrolysis The cathode increases in mass while the anode decreases. Positive ions are attracted to the cathode, where they pick up one or more electrons and are discharged. Either the metal is deposited or you get hydrogen. When a steady current is passed through the solution, the net result is that silver metal is removed from the anode and deposited on. What Happens At The Cathode And Anode During Electrolysis.
From www.slideserve.com
PPT ELECTROLYSIS A guide for GCSE students PowerPoint Presentation What Happens At The Cathode And Anode During Electrolysis Positive ions, cations close cation an atom or group of atoms that have lost electrons and become positively charged. What happens at the cathode? The positive anode attracts anions toward it, while the negative. The power source pumps the new electrons on the anode along the circuit to replace the ones taken off the cathode. Either the metal is deposited. What Happens At The Cathode And Anode During Electrolysis.
From mavink.com
Anode Cathode Diagram What Happens At The Cathode And Anode During Electrolysis When a steady current is passed through the solution, the net result is that silver metal is removed from the anode and deposited on the cathode. The cathode increases in mass while the anode decreases. The power source pumps the new electrons on the anode along the circuit to replace the ones taken off the cathode. The positive anode attracts. What Happens At The Cathode And Anode During Electrolysis.
From www.edplace.com
Understand How Electrolysis Works Worksheet EdPlace What Happens At The Cathode And Anode During Electrolysis The table summarises the product formed at the anode during the electrolysis of different electrolytes close electrolyte a substance. When a steady current is passed through the solution, the net result is that silver metal is removed from the anode and deposited on the cathode. What happens at the cathode? This occurs as copper atoms are oxidised at the anode. What Happens At The Cathode And Anode During Electrolysis.
From enginelibsaprozoic.z21.web.core.windows.net
What Happens At The Cathode In Electrolysis What Happens At The Cathode And Anode During Electrolysis The positive anode attracts anions toward it, while the negative. Positive ions, cations close cation an atom or group of atoms that have lost electrons and become positively charged. The power source pumps the new electrons on the anode along the circuit to replace the ones taken off the cathode. This occurs as copper atoms are oxidised at the anode. What Happens At The Cathode And Anode During Electrolysis.
From www.researchgate.net
Figure The anode and cathode reactions in typical electrolytic What Happens At The Cathode And Anode During Electrolysis The battery pumps electrons away from the anode (making it positive) and into the cathode (making it negative). This occurs as copper atoms are oxidised at the anode and form ions while copper ions are reduced at the. The power source pumps the new electrons on the anode along the circuit to replace the ones taken off the cathode. The. What Happens At The Cathode And Anode During Electrolysis.
From byjus.com
In the electrolytic refining of a metal “M”, what would you take as the What Happens At The Cathode And Anode During Electrolysis The cathode increases in mass while the anode decreases. Positive ions, cations close cation an atom or group of atoms that have lost electrons and become positively charged. The power source pumps the new electrons on the anode along the circuit to replace the ones taken off the cathode. Either the metal is deposited or you get hydrogen. At the. What Happens At The Cathode And Anode During Electrolysis.
From fyodeauzx.blob.core.windows.net
Electrode Effect Electrolysis at Edna Boardman blog What Happens At The Cathode And Anode During Electrolysis The table summarises the product formed at the anode during the electrolysis of different electrolytes close electrolyte a substance. When a steady current is passed through the solution, the net result is that silver metal is removed from the anode and deposited on the cathode. What happens at the cathode? Positive ions are attracted to the cathode, where they pick. What Happens At The Cathode And Anode During Electrolysis.