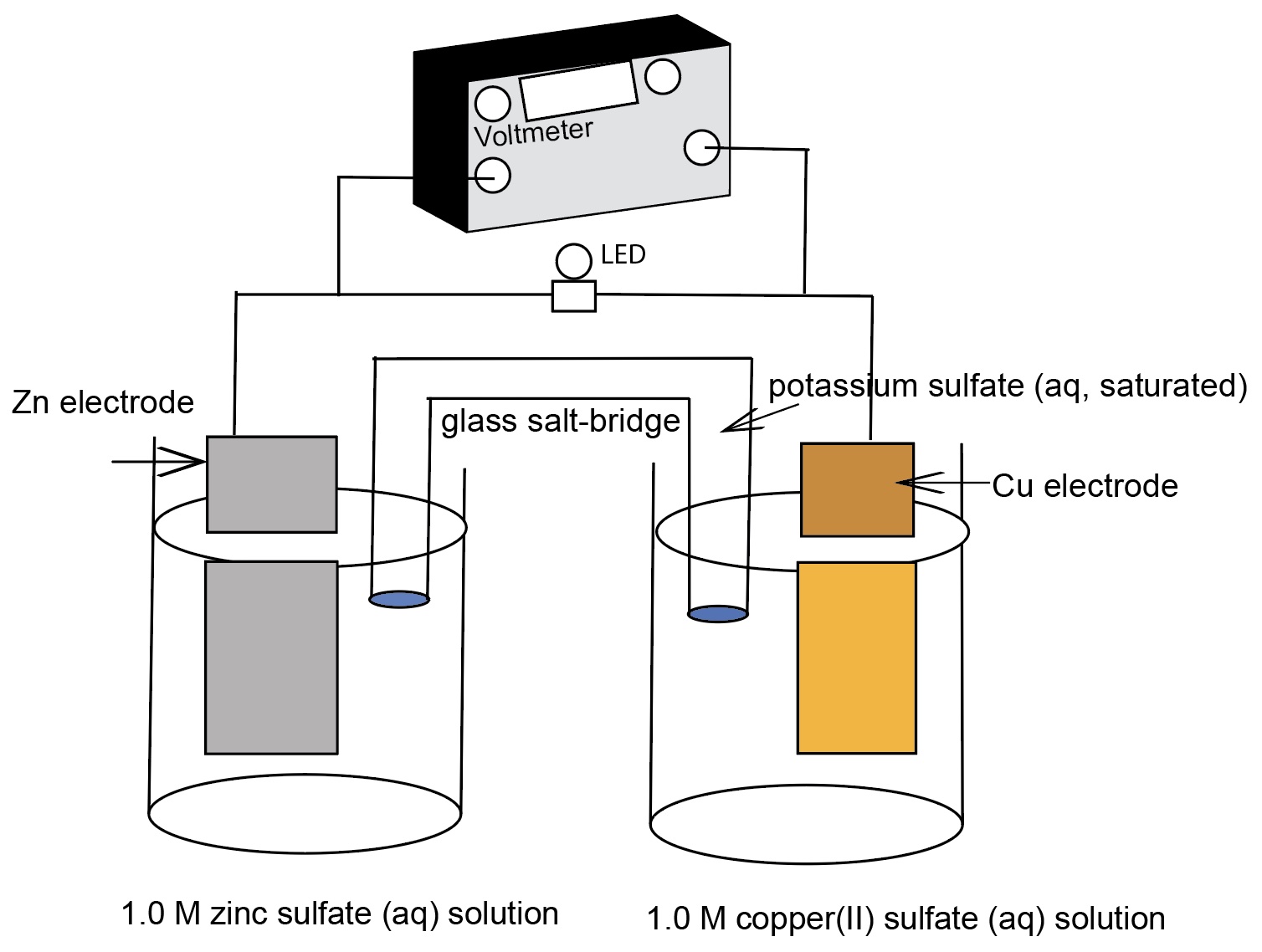
Galvanic Cell Negative Voltage . a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (δg < 0) to generate electricity. in this section, we focus on reactions that occur in galvanic cells. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. the positive voltage for eo cell indicates that at standard conditions the reaction is spontaneous. to measure the potential of the cu/cu 2 + couple, we can construct a galvanic cell analogous to the one shown in figure. Recall that ∆go = − nfeo. The theoretical voltage is given by the nernst equation. This type of electrochemical cell is. to measure the energy produced in a galvanic cell, we will use a voltmeter, placing it in the pathway of the.
from ar.inspiredpencil.com
a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (δg < 0) to generate electricity. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. The theoretical voltage is given by the nernst equation. in this section, we focus on reactions that occur in galvanic cells. This type of electrochemical cell is. to measure the energy produced in a galvanic cell, we will use a voltmeter, placing it in the pathway of the. Recall that ∆go = − nfeo. to measure the potential of the cu/cu 2 + couple, we can construct a galvanic cell analogous to the one shown in figure. the positive voltage for eo cell indicates that at standard conditions the reaction is spontaneous.
Galvanic Cell Labeled
Galvanic Cell Negative Voltage a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (δg < 0) to generate electricity. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. in this section, we focus on reactions that occur in galvanic cells. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (δg < 0) to generate electricity. to measure the potential of the cu/cu 2 + couple, we can construct a galvanic cell analogous to the one shown in figure. to measure the energy produced in a galvanic cell, we will use a voltmeter, placing it in the pathway of the. The theoretical voltage is given by the nernst equation. the positive voltage for eo cell indicates that at standard conditions the reaction is spontaneous. This type of electrochemical cell is. Recall that ∆go = − nfeo.
From www.slideserve.com
PPT Galvanic Cells PowerPoint Presentation, free download ID5404902 Galvanic Cell Negative Voltage to measure the potential of the cu/cu 2 + couple, we can construct a galvanic cell analogous to the one shown in figure. Recall that ∆go = − nfeo. to measure the energy produced in a galvanic cell, we will use a voltmeter, placing it in the pathway of the. a galvanic (voltaic) cell uses the energy. Galvanic Cell Negative Voltage.
From byjus.com
is cathode negative terminal or positive terminal? why it is different Galvanic Cell Negative Voltage a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (δg < 0) to generate electricity. the positive voltage for eo cell indicates that at standard conditions the reaction is spontaneous. The theoretical voltage is given by the nernst equation. to measure the energy produced in a galvanic cell, we will use a voltmeter,. Galvanic Cell Negative Voltage.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps Galvanic Cell Negative Voltage This type of electrochemical cell is. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (δg < 0) to generate electricity. in this section, we focus on reactions that occur in galvanic cells. Recall that ∆go = − nfeo. to measure the energy produced in a galvanic cell, we will use a voltmeter,. Galvanic Cell Negative Voltage.
From www.youtube.com
Calculating Voltage of Galvanic Cell YouTube Galvanic Cell Negative Voltage the positive voltage for eo cell indicates that at standard conditions the reaction is spontaneous. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. Recall that ∆go = − nfeo. This type of electrochemical cell is. in this section, we focus on reactions that occur in. Galvanic Cell Negative Voltage.
From saylordotorg.github.io
Electrochemistry Galvanic Cell Negative Voltage the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. This type of electrochemical cell is. The theoretical voltage is given by the nernst equation. to measure the potential of the cu/cu 2 + couple, we can construct a galvanic cell analogous to the one shown in figure.. Galvanic Cell Negative Voltage.
From courses.lumenlearning.com
Galvanic Cells Chemistry Galvanic Cell Negative Voltage in this section, we focus on reactions that occur in galvanic cells. The theoretical voltage is given by the nernst equation. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. to measure the energy produced in a galvanic cell, we will use a voltmeter, placing it. Galvanic Cell Negative Voltage.
From www.scienceabc.com
What Are Galvanic Cells? An Oversimplified Explanation » ScienceABC Galvanic Cell Negative Voltage to measure the energy produced in a galvanic cell, we will use a voltmeter, placing it in the pathway of the. This type of electrochemical cell is. The theoretical voltage is given by the nernst equation. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a. Galvanic Cell Negative Voltage.
From byjus.com
What is the free energy change for galvanic cell? Galvanic Cell Negative Voltage in this section, we focus on reactions that occur in galvanic cells. to measure the energy produced in a galvanic cell, we will use a voltmeter, placing it in the pathway of the. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (δg < 0) to generate electricity. the positive voltage for. Galvanic Cell Negative Voltage.
From www.vecteezy.com
Voltaic galvanic cell or daniell cell.Redox reaction.Oxidation and Galvanic Cell Negative Voltage a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (δg < 0) to generate electricity. The theoretical voltage is given by the nernst equation. to measure the energy produced in a galvanic cell, we will use a voltmeter, placing it in the pathway of the. the galvanic cell, or called voltaic cell, is. Galvanic Cell Negative Voltage.
From scienceinfo.com
Galvanic Cell (Voltaic Cell) Definition, Working Principle Galvanic Cell Negative Voltage This type of electrochemical cell is. Recall that ∆go = − nfeo. to measure the energy produced in a galvanic cell, we will use a voltmeter, placing it in the pathway of the. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a galvanic (voltaic) cell. Galvanic Cell Negative Voltage.
From chem.libretexts.org
1 Electrochemical Cells (Experiment) Chemistry LibreTexts Galvanic Cell Negative Voltage to measure the potential of the cu/cu 2 + couple, we can construct a galvanic cell analogous to the one shown in figure. The theoretical voltage is given by the nernst equation. the positive voltage for eo cell indicates that at standard conditions the reaction is spontaneous. the galvanic cell, or called voltaic cell, is an electrochemical. Galvanic Cell Negative Voltage.
From www.slideserve.com
PPT Oxidation and Reduction Reactions PowerPoint Presentation, free Galvanic Cell Negative Voltage in this section, we focus on reactions that occur in galvanic cells. to measure the potential of the cu/cu 2 + couple, we can construct a galvanic cell analogous to the one shown in figure. to measure the energy produced in a galvanic cell, we will use a voltmeter, placing it in the pathway of the. Recall. Galvanic Cell Negative Voltage.
From slideplayer.com
Electrochemistry Movement of Electrons Chemistry of Metal Reactions Galvanic Cell Negative Voltage in this section, we focus on reactions that occur in galvanic cells. Recall that ∆go = − nfeo. to measure the energy produced in a galvanic cell, we will use a voltmeter, placing it in the pathway of the. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical. Galvanic Cell Negative Voltage.
From www.jove.com
Voltaic/Galvanic Cells Principle, Components, Cell Notation JoVE Galvanic Cell Negative Voltage to measure the energy produced in a galvanic cell, we will use a voltmeter, placing it in the pathway of the. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. This type of electrochemical cell is. Recall that ∆go = − nfeo. to measure the potential. Galvanic Cell Negative Voltage.
From biochemreview.weebly.com
Galvanic cell BIOChemReview Galvanic Cell Negative Voltage the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (δg < 0) to generate electricity. The theoretical voltage is given by the nernst equation. in this section, we focus on reactions that occur. Galvanic Cell Negative Voltage.
From chemistrytalk.org
Galvanic Cells & Voltaic Cells Electrochemical Cells ChemTalk Galvanic Cell Negative Voltage the positive voltage for eo cell indicates that at standard conditions the reaction is spontaneous. The theoretical voltage is given by the nernst equation. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (δg < 0) to generate electricity. This type of electrochemical cell is. the galvanic cell, or called voltaic cell, is. Galvanic Cell Negative Voltage.
From stock.adobe.com
Galvanic voltaic cell infographic diagram battery part structure Galvanic Cell Negative Voltage to measure the potential of the cu/cu 2 + couple, we can construct a galvanic cell analogous to the one shown in figure. Recall that ∆go = − nfeo. The theoretical voltage is given by the nernst equation. This type of electrochemical cell is. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (δg. Galvanic Cell Negative Voltage.
From ar.inspiredpencil.com
Galvanic Cell Labeled Galvanic Cell Negative Voltage the positive voltage for eo cell indicates that at standard conditions the reaction is spontaneous. Recall that ∆go = − nfeo. in this section, we focus on reactions that occur in galvanic cells. This type of electrochemical cell is. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical. Galvanic Cell Negative Voltage.
From cider.uoregon.edu
Voltaic Galvanic Cell (Electrochemical Cell) Simulation AACT CIDER Galvanic Cell Negative Voltage a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (δg < 0) to generate electricity. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. the positive voltage for eo cell indicates that at standard conditions the reaction is spontaneous. to measure. Galvanic Cell Negative Voltage.
From studybreathings.z21.web.core.windows.net
How Do Electrons Flow In A Galvanic Cell Galvanic Cell Negative Voltage This type of electrochemical cell is. Recall that ∆go = − nfeo. the positive voltage for eo cell indicates that at standard conditions the reaction is spontaneous. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (δg < 0) to generate electricity. to measure the potential of the cu/cu 2 + couple, we. Galvanic Cell Negative Voltage.
From www.nanoscience.com
Electrochemistry Nanoscience Instruments Galvanic Cell Negative Voltage The theoretical voltage is given by the nernst equation. This type of electrochemical cell is. in this section, we focus on reactions that occur in galvanic cells. the positive voltage for eo cell indicates that at standard conditions the reaction is spontaneous. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (δg <. Galvanic Cell Negative Voltage.
From dxodcnqen.blob.core.windows.net
Standard Hydrogen Electrode In A Galvanic Cell at Katharine Murphy blog Galvanic Cell Negative Voltage a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (δg < 0) to generate electricity. The theoretical voltage is given by the nernst equation. This type of electrochemical cell is. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. to measure the. Galvanic Cell Negative Voltage.
From glossary.periodni.com
Galvanic cell Chemistry Dictionary & Glossary Galvanic Cell Negative Voltage This type of electrochemical cell is. the positive voltage for eo cell indicates that at standard conditions the reaction is spontaneous. to measure the energy produced in a galvanic cell, we will use a voltmeter, placing it in the pathway of the. in this section, we focus on reactions that occur in galvanic cells. the galvanic. Galvanic Cell Negative Voltage.
From stock.adobe.com
Vector scientific illustration of electrolysis process with a bulb Galvanic Cell Negative Voltage in this section, we focus on reactions that occur in galvanic cells. The theoretical voltage is given by the nernst equation. This type of electrochemical cell is. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (δg < 0) to generate electricity. Recall that ∆go = − nfeo. to measure the potential of. Galvanic Cell Negative Voltage.
From kemilektioner.se
Test Galvaniska element Galvanic Cell Negative Voltage a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (δg < 0) to generate electricity. This type of electrochemical cell is. in this section, we focus on reactions that occur in galvanic cells. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from.. Galvanic Cell Negative Voltage.
From chemistry.stackexchange.com
physical chemistry Positive or Negative Anode/Cathode in Electrolytic Galvanic Cell Negative Voltage to measure the potential of the cu/cu 2 + couple, we can construct a galvanic cell analogous to the one shown in figure. The theoretical voltage is given by the nernst equation. the positive voltage for eo cell indicates that at standard conditions the reaction is spontaneous. in this section, we focus on reactions that occur in. Galvanic Cell Negative Voltage.
From www.scienceabc.com
Galvanic Cell Definition, Diagram And Working Galvanic Cell Negative Voltage the positive voltage for eo cell indicates that at standard conditions the reaction is spontaneous. The theoretical voltage is given by the nernst equation. to measure the potential of the cu/cu 2 + couple, we can construct a galvanic cell analogous to the one shown in figure. This type of electrochemical cell is. a galvanic (voltaic) cell. Galvanic Cell Negative Voltage.
From 2012books.lardbucket.org
Electrochemistry Galvanic Cell Negative Voltage to measure the energy produced in a galvanic cell, we will use a voltmeter, placing it in the pathway of the. in this section, we focus on reactions that occur in galvanic cells. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from. The theoretical voltage is. Galvanic Cell Negative Voltage.
From www.chegg.com
Solved Calculate the voltage produced by a galvanic cell Galvanic Cell Negative Voltage The theoretical voltage is given by the nernst equation. in this section, we focus on reactions that occur in galvanic cells. This type of electrochemical cell is. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (δg < 0) to generate electricity. to measure the energy produced in a galvanic cell, we will. Galvanic Cell Negative Voltage.
From chem.eduinsightful.com
Galvanic Cells Decoded Igniting a Spark of Knowledge Galvanic Cell Negative Voltage The theoretical voltage is given by the nernst equation. in this section, we focus on reactions that occur in galvanic cells. to measure the potential of the cu/cu 2 + couple, we can construct a galvanic cell analogous to the one shown in figure. a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction. Galvanic Cell Negative Voltage.
From studybreathings.z21.web.core.windows.net
How Do Electrons Flow In A Galvanic Cell Galvanic Cell Negative Voltage to measure the potential of the cu/cu 2 + couple, we can construct a galvanic cell analogous to the one shown in figure. This type of electrochemical cell is. to measure the energy produced in a galvanic cell, we will use a voltmeter, placing it in the pathway of the. the positive voltage for eo cell indicates. Galvanic Cell Negative Voltage.
From www.youtube.com
How To Draw Galvanic Cells and Voltaic Cells Electrochemistry YouTube Galvanic Cell Negative Voltage This type of electrochemical cell is. The theoretical voltage is given by the nernst equation. Recall that ∆go = − nfeo. to measure the energy produced in a galvanic cell, we will use a voltmeter, placing it in the pathway of the. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy. Galvanic Cell Negative Voltage.
From general.chemistrysteps.com
Galvanic Cells Chemistry Steps Galvanic Cell Negative Voltage This type of electrochemical cell is. The theoretical voltage is given by the nernst equation. to measure the potential of the cu/cu 2 + couple, we can construct a galvanic cell analogous to the one shown in figure. the galvanic cell, or called voltaic cell, is an electrochemical cell that converts the chemical energy to electrical energy from.. Galvanic Cell Negative Voltage.
From www.geeksforgeeks.org
Galvanic Cell Definition, Construction, Working Principle Galvanic Cell Negative Voltage The theoretical voltage is given by the nernst equation. in this section, we focus on reactions that occur in galvanic cells. This type of electrochemical cell is. to measure the potential of the cu/cu 2 + couple, we can construct a galvanic cell analogous to the one shown in figure. a galvanic (voltaic) cell uses the energy. Galvanic Cell Negative Voltage.
From www.slideserve.com
PPT Voltaic/Galvanic Cells PowerPoint Presentation, free download Galvanic Cell Negative Voltage a galvanic (voltaic) cell uses the energy released during a spontaneous redox reaction (δg < 0) to generate electricity. Recall that ∆go = − nfeo. the positive voltage for eo cell indicates that at standard conditions the reaction is spontaneous. to measure the potential of the cu/cu 2 + couple, we can construct a galvanic cell analogous. Galvanic Cell Negative Voltage.