Lead Chloride Reacts With Cl2 To Give Pbcl4 . Pbcl 2 is a reducing agent (i.e. rationalise the given statements and give chemical reactions: Lead (ii) chloride reacts with cl2 to give. • lead (ii) chloride reacts with cl2 to give pbcl4. (b) lead (iv) chloride is highly unstable towards heat. (a) lead(ii) chloride reacts with c l 2 to give p b c l 4. Lead (ii) chloride reacts with cl2 to give pbcl4. Get your questions answered by the expert for free. Pbcl2 + cl2 = pbcl4 is a redox reaction where pb is oxidized and cl is reduced. (a) lead (ii) chloride reacts with cl 2 to give pbcl 4. Rationalise the given statements and give chemical reactions: summary of redox reaction. (b) lead(iv) chloride is highly unstable towards heat. explore detailed answers to question 13 rationalise the given statements and give chemical reactions :
from www.911metallurgist.com
Lead (ii) chloride reacts with cl2 to give pbcl4. (b) lead(iv) chloride is highly unstable towards heat. (b) lead (iv) chloride is highly unstable towards heat. Pbcl 2 is a reducing agent (i.e. Rationalise the given statements and give chemical reactions: • lead (ii) chloride reacts with cl2 to give pbcl4. summary of redox reaction. Pbcl2 + cl2 = pbcl4 is a redox reaction where pb is oxidized and cl is reduced. Get your questions answered by the expert for free. (a) lead (ii) chloride reacts with cl 2 to give pbcl 4.
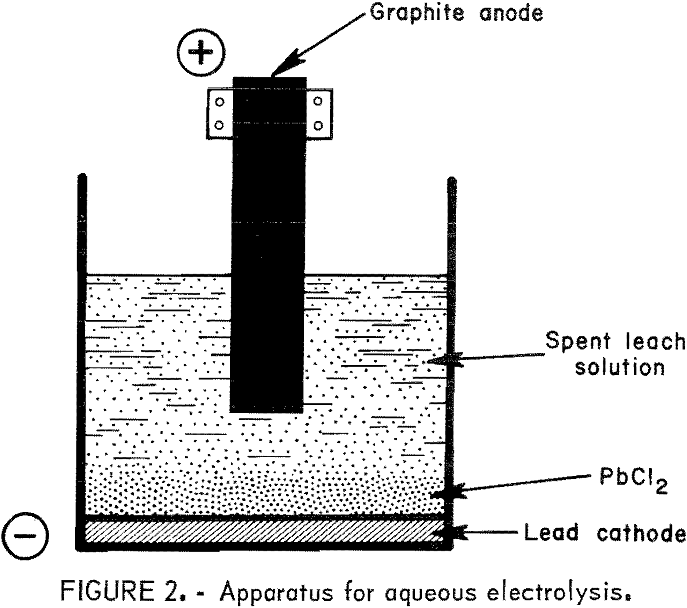
Lead Chloride Electrolysis
Lead Chloride Reacts With Cl2 To Give Pbcl4 (a) lead(ii) chloride reacts with c l 2 to give p b c l 4. (a) lead (ii) chloride reacts with cl 2 to give pbcl 4. explore detailed answers to question 13 rationalise the given statements and give chemical reactions : (a) lead(ii) chloride reacts with c l 2 to give p b c l 4. Pbcl 2 is a reducing agent (i.e. Get your questions answered by the expert for free. (b) lead(iv) chloride is highly unstable towards heat. (b) lead (iv) chloride is highly unstable towards heat. Rationalise the given statements and give chemical reactions: • lead (ii) chloride reacts with cl2 to give pbcl4. Lead (ii) chloride reacts with cl2 to give. summary of redox reaction. Lead (ii) chloride reacts with cl2 to give pbcl4. rationalise the given statements and give chemical reactions: Pbcl2 + cl2 = pbcl4 is a redox reaction where pb is oxidized and cl is reduced.
From dir.indiamart.com
Lead Chloride PbCl2 Latest Price, Manufacturers & Suppliers Lead Chloride Reacts With Cl2 To Give Pbcl4 Pbcl 2 is a reducing agent (i.e. Get your questions answered by the expert for free. (b) lead(iv) chloride is highly unstable towards heat. summary of redox reaction. Lead (ii) chloride reacts with cl2 to give. explore detailed answers to question 13 rationalise the given statements and give chemical reactions : rationalise the given statements and give. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From courses.lumenlearning.com
4.2 Classifying Chemical Reactions Chemistry Lead Chloride Reacts With Cl2 To Give Pbcl4 explore detailed answers to question 13 rationalise the given statements and give chemical reactions : rationalise the given statements and give chemical reactions: (b) lead (iv) chloride is highly unstable towards heat. Get your questions answered by the expert for free. Lead (ii) chloride reacts with cl2 to give pbcl4. (a) lead (ii) chloride reacts with cl. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.youtube.com
Cl_ 2 on reaction with hot & conc. NaOH gives two chlorine having Lead Chloride Reacts With Cl2 To Give Pbcl4 rationalise the given statements and give chemical reactions: Lead (ii) chloride reacts with cl2 to give. explore detailed answers to question 13 rationalise the given statements and give chemical reactions : Rationalise the given statements and give chemical reactions: Get your questions answered by the expert for free. (b) lead (iv) chloride is highly unstable towards heat. . Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.numerade.com
SOLVED Question 23 (3 points) Calculate the number of moles of Lead Chloride Reacts With Cl2 To Give Pbcl4 Rationalise the given statements and give chemical reactions: explore detailed answers to question 13 rationalise the given statements and give chemical reactions : summary of redox reaction. (b) lead (iv) chloride is highly unstable towards heat. rationalise the given statements and give chemical reactions: (a) lead (ii) chloride reacts with cl 2 to give pbcl 4.. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.fishersci.no
Lead(II) chloride, Reagent Grade, 99, Thermo Scientific Chemicals Lead Chloride Reacts With Cl2 To Give Pbcl4 (b) lead (iv) chloride is highly unstable towards heat. summary of redox reaction. Pbcl2 + cl2 = pbcl4 is a redox reaction where pb is oxidized and cl is reduced. • lead (ii) chloride reacts with cl2 to give pbcl4. (a) lead(ii) chloride reacts with c l 2 to give p b c l 4. explore detailed. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From encyclopedia.pub
Compounds of Lead Encyclopedia MDPI Lead Chloride Reacts With Cl2 To Give Pbcl4 Lead (ii) chloride reacts with cl2 to give pbcl4. (b) lead (iv) chloride is highly unstable towards heat. explore detailed answers to question 13 rationalise the given statements and give chemical reactions : Pbcl 2 is a reducing agent (i.e. Lead (ii) chloride reacts with cl2 to give. (a) lead(ii) chloride reacts with c l 2 to give. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.youtube.com
Equation for PbCl2 + H2O Lead (II) chloride + Water YouTube Lead Chloride Reacts With Cl2 To Give Pbcl4 (a) lead(ii) chloride reacts with c l 2 to give p b c l 4. Rationalise the given statements and give chemical reactions: (b) lead(iv) chloride is highly unstable towards heat. Pbcl 2 is a reducing agent (i.e. (a) lead (ii) chloride reacts with cl 2 to give pbcl 4. explore detailed answers to question 13 rationalise. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From byjus.com
when propene reacts with CL2 in light gives A . A reacts with chlorine Lead Chloride Reacts With Cl2 To Give Pbcl4 • lead (ii) chloride reacts with cl2 to give pbcl4. Lead (ii) chloride reacts with cl2 to give. (a) lead(ii) chloride reacts with c l 2 to give p b c l 4. Pbcl2 + cl2 = pbcl4 is a redox reaction where pb is oxidized and cl is reduced. explore detailed answers to question 13 rationalise the. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From guides.byjusweb.com
Lead II Chloride Formula Definition, Concepts and Examples Lead Chloride Reacts With Cl2 To Give Pbcl4 Rationalise the given statements and give chemical reactions: Pbcl 2 is a reducing agent (i.e. Pbcl2 + cl2 = pbcl4 is a redox reaction where pb is oxidized and cl is reduced. summary of redox reaction. (a) lead (ii) chloride reacts with cl 2 to give pbcl 4. rationalise the given statements and give chemical reactions: Lead. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.911metallurgist.com
Lead Chloride Electrolysis Lead Chloride Reacts With Cl2 To Give Pbcl4 (b) lead(iv) chloride is highly unstable towards heat. (a) lead (ii) chloride reacts with cl 2 to give pbcl 4. • lead (ii) chloride reacts with cl2 to give pbcl4. rationalise the given statements and give chemical reactions: Pbcl2 + cl2 = pbcl4 is a redox reaction where pb is oxidized and cl is reduced. Pbcl 2 is. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.youtube.com
How to Write the Net Ionic Equation for Pb(NO3)2 + NH4Cl = PbCl2 Lead Chloride Reacts With Cl2 To Give Pbcl4 (b) lead(iv) chloride is highly unstable towards heat. Rationalise the given statements and give chemical reactions: explore detailed answers to question 13 rationalise the given statements and give chemical reactions : Lead (ii) chloride reacts with cl2 to give. Pbcl 2 is a reducing agent (i.e. Lead (ii) chloride reacts with cl2 to give pbcl4. (b) lead (iv) chloride. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.numerade.com
SOLVED Write the reactions for the formation of the three chlorides in Lead Chloride Reacts With Cl2 To Give Pbcl4 • lead (ii) chloride reacts with cl2 to give pbcl4. Lead (ii) chloride reacts with cl2 to give. (a) lead(ii) chloride reacts with c l 2 to give p b c l 4. summary of redox reaction. explore detailed answers to question 13 rationalise the given statements and give chemical reactions : Lead (ii) chloride reacts with. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.slideserve.com
PPT Ionic Compounds Formula to Name PowerPoint Presentation, free Lead Chloride Reacts With Cl2 To Give Pbcl4 Rationalise the given statements and give chemical reactions: rationalise the given statements and give chemical reactions: Pbcl 2 is a reducing agent (i.e. (b) lead (iv) chloride is highly unstable towards heat. (a) lead(ii) chloride reacts with c l 2 to give p b c l 4. Lead (ii) chloride reacts with cl2 to give pbcl4. (b) lead(iv). Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.numerade.com
When lead chloride (PbClz) is placed in otherwise pure water; enough Lead Chloride Reacts With Cl2 To Give Pbcl4 explore detailed answers to question 13 rationalise the given statements and give chemical reactions : Pbcl 2 is a reducing agent (i.e. Pbcl2 + cl2 = pbcl4 is a redox reaction where pb is oxidized and cl is reduced. • lead (ii) chloride reacts with cl2 to give pbcl4. (b) lead(iv) chloride is highly unstable towards heat. Lead (ii). Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.911metallurgist.com
How to Recover Lead from Lead Chloride by Electrolysis Lead Chloride Reacts With Cl2 To Give Pbcl4 rationalise the given statements and give chemical reactions: Get your questions answered by the expert for free. Pbcl 2 is a reducing agent (i.e. summary of redox reaction. (b) lead (iv) chloride is highly unstable towards heat. Lead (ii) chloride reacts with cl2 to give pbcl4. Lead (ii) chloride reacts with cl2 to give. (a) lead(ii) chloride. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.numerade.com
SOLVED Methane reacts with chlorine in the presence of ultraviolet Lead Chloride Reacts With Cl2 To Give Pbcl4 Get your questions answered by the expert for free. rationalise the given statements and give chemical reactions: Rationalise the given statements and give chemical reactions: Pbcl2 + cl2 = pbcl4 is a redox reaction where pb is oxidized and cl is reduced. (b) lead (iv) chloride is highly unstable towards heat. (b) lead(iv) chloride is highly unstable towards heat.. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.numerade.com
SOLVED You are given the following information PbCl2 PbCl2 AH = 329. Lead Chloride Reacts With Cl2 To Give Pbcl4 explore detailed answers to question 13 rationalise the given statements and give chemical reactions : • lead (ii) chloride reacts with cl2 to give pbcl4. Pbcl2 + cl2 = pbcl4 is a redox reaction where pb is oxidized and cl is reduced. Lead (ii) chloride reacts with cl2 to give pbcl4. (a) lead(ii) chloride reacts with c l. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.youtube.com
Lead(II) Chloride Synthesis (PbCl2) YouTube Lead Chloride Reacts With Cl2 To Give Pbcl4 Pbcl2 + cl2 = pbcl4 is a redox reaction where pb is oxidized and cl is reduced. rationalise the given statements and give chemical reactions: explore detailed answers to question 13 rationalise the given statements and give chemical reactions : (a) lead(ii) chloride reacts with c l 2 to give p b c l 4. • lead. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.numerade.com
SOLVEDAqueous aluminum chloride reacts with a lead (II) nitrate Lead Chloride Reacts With Cl2 To Give Pbcl4 explore detailed answers to question 13 rationalise the given statements and give chemical reactions : Rationalise the given statements and give chemical reactions: Lead (ii) chloride reacts with cl2 to give. (a) lead (ii) chloride reacts with cl 2 to give pbcl 4. summary of redox reaction. rationalise the given statements and give chemical reactions: Pbcl. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.911metallurgist.com
Lead Chloride Electrolysis Lead Chloride Reacts With Cl2 To Give Pbcl4 (a) lead (ii) chloride reacts with cl 2 to give pbcl 4. (b) lead(iv) chloride is highly unstable towards heat. Get your questions answered by the expert for free. explore detailed answers to question 13 rationalise the given statements and give chemical reactions : Lead (ii) chloride reacts with cl2 to give. Rationalise the given statements and give. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From collegedunia.com
Lead II Chloride Formula Properties, Structure, Examples Lead Chloride Reacts With Cl2 To Give Pbcl4 rationalise the given statements and give chemical reactions: (a) lead (ii) chloride reacts with cl 2 to give pbcl 4. (a) lead(ii) chloride reacts with c l 2 to give p b c l 4. Rationalise the given statements and give chemical reactions: explore detailed answers to question 13 rationalise the given statements and give chemical. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.numerade.com
SOLVEDLead(IV) oxide is a strong oxidizing agent. For example, lead(IV Lead Chloride Reacts With Cl2 To Give Pbcl4 Get your questions answered by the expert for free. (b) lead (iv) chloride is highly unstable towards heat. Lead (ii) chloride reacts with cl2 to give pbcl4. Rationalise the given statements and give chemical reactions: (a) lead (ii) chloride reacts with cl 2 to give pbcl 4. Pbcl2 + cl2 = pbcl4 is a redox reaction where pb is. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.chemistryscl.com
Benzene and Chlorine Reaction C6H6 + Cl2, Mechanism Lead Chloride Reacts With Cl2 To Give Pbcl4 summary of redox reaction. Lead (ii) chloride reacts with cl2 to give pbcl4. (b) lead(iv) chloride is highly unstable towards heat. rationalise the given statements and give chemical reactions: • lead (ii) chloride reacts with cl2 to give pbcl4. Lead (ii) chloride reacts with cl2 to give. (a) lead (ii) chloride reacts with cl 2 to give. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.slideserve.com
PPT Formula writing PowerPoint Presentation, free download ID4565553 Lead Chloride Reacts With Cl2 To Give Pbcl4 rationalise the given statements and give chemical reactions: explore detailed answers to question 13 rationalise the given statements and give chemical reactions : Lead (ii) chloride reacts with cl2 to give. (b) lead (iv) chloride is highly unstable towards heat. (a) lead(ii) chloride reacts with c l 2 to give p b c l 4. (a). Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.numerade.com
SOLVED Chlorine gas reacts with fluorine gas to form chlorine Lead Chloride Reacts With Cl2 To Give Pbcl4 Get your questions answered by the expert for free. (a) lead (ii) chloride reacts with cl 2 to give pbcl 4. (a) lead(ii) chloride reacts with c l 2 to give p b c l 4. summary of redox reaction. Rationalise the given statements and give chemical reactions: Lead (ii) chloride reacts with cl2 to give. (b). Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.wikidoc.org
Lead wikidoc Lead Chloride Reacts With Cl2 To Give Pbcl4 (b) lead(iv) chloride is highly unstable towards heat. (a) lead(ii) chloride reacts with c l 2 to give p b c l 4. Lead (ii) chloride reacts with cl2 to give. (a) lead (ii) chloride reacts with cl 2 to give pbcl 4. Pbcl2 + cl2 = pbcl4 is a redox reaction where pb is oxidized and cl. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.911metallurgist.com
Lead Chloride Electrolysis Lead Chloride Reacts With Cl2 To Give Pbcl4 Get your questions answered by the expert for free. Pbcl 2 is a reducing agent (i.e. Lead (ii) chloride reacts with cl2 to give. (b) lead(iv) chloride is highly unstable towards heat. Pbcl2 + cl2 = pbcl4 is a redox reaction where pb is oxidized and cl is reduced. summary of redox reaction. (a) lead(ii) chloride reacts with. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.alamy.com
PbCl4 lead(IV) chloride CAS 13463304 chemical substance in white Lead Chloride Reacts With Cl2 To Give Pbcl4 Rationalise the given statements and give chemical reactions: rationalise the given statements and give chemical reactions: explore detailed answers to question 13 rationalise the given statements and give chemical reactions : Lead (ii) chloride reacts with cl2 to give. • lead (ii) chloride reacts with cl2 to give pbcl4. (a) lead(ii) chloride reacts with c l 2. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.youtube.com
Cl2 Lewis Structure How to Draw the Dot Structure for Cl2 YouTube Lead Chloride Reacts With Cl2 To Give Pbcl4 • lead (ii) chloride reacts with cl2 to give pbcl4. Lead (ii) chloride reacts with cl2 to give pbcl4. Lead (ii) chloride reacts with cl2 to give. Pbcl2 + cl2 = pbcl4 is a redox reaction where pb is oxidized and cl is reduced. rationalise the given statements and give chemical reactions: (b) lead(iv) chloride is highly unstable towards. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.youtube.com
Reaction of Sodium with Chlorine (Na+Cl2) YouTube Lead Chloride Reacts With Cl2 To Give Pbcl4 Pbcl2 + cl2 = pbcl4 is a redox reaction where pb is oxidized and cl is reduced. (a) lead(ii) chloride reacts with c l 2 to give p b c l 4. (b) lead (iv) chloride is highly unstable towards heat. Lead (ii) chloride reacts with cl2 to give pbcl4. summary of redox reaction. Get your questions answered. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.numerade.com
SOLVED The name of the compound PbCl4 is Lead tetrachloride Lead(II Lead Chloride Reacts With Cl2 To Give Pbcl4 (b) lead(iv) chloride is highly unstable towards heat. rationalise the given statements and give chemical reactions: explore detailed answers to question 13 rationalise the given statements and give chemical reactions : • lead (ii) chloride reacts with cl2 to give pbcl4. (a) lead (ii) chloride reacts with cl 2 to give pbcl 4. Rationalise the given statements. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.slideserve.com
PPT PRECIPITATION REACTIONS Solubility of Salts Section 18.4 Lead Chloride Reacts With Cl2 To Give Pbcl4 (b) lead(iv) chloride is highly unstable towards heat. Pbcl2 + cl2 = pbcl4 is a redox reaction where pb is oxidized and cl is reduced. (a) lead(ii) chloride reacts with c l 2 to give p b c l 4. • lead (ii) chloride reacts with cl2 to give pbcl4. rationalise the given statements and give chemical reactions:. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.chegg.com
Solved Study this chemical reaction Pb + 2Cl2 = PbCl4 Lead Chloride Reacts With Cl2 To Give Pbcl4 Lead (ii) chloride reacts with cl2 to give pbcl4. (a) lead (ii) chloride reacts with cl 2 to give pbcl 4. Rationalise the given statements and give chemical reactions: Pbcl 2 is a reducing agent (i.e. rationalise the given statements and give chemical reactions: (a) lead(ii) chloride reacts with c l 2 to give p b c. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From dxoilsdmm.blob.core.windows.net
Urine And Chlorine Reaction at Lynn Quinones blog Lead Chloride Reacts With Cl2 To Give Pbcl4 Pbcl 2 is a reducing agent (i.e. (b) lead (iv) chloride is highly unstable towards heat. Lead (ii) chloride reacts with cl2 to give. (a) lead (ii) chloride reacts with cl 2 to give pbcl 4. Pbcl2 + cl2 = pbcl4 is a redox reaction where pb is oxidized and cl is reduced. rationalise the given statements and. Lead Chloride Reacts With Cl2 To Give Pbcl4.
From www.numerade.com
SOLVEDChlorine reacts with chloroform according to the reaction given Lead Chloride Reacts With Cl2 To Give Pbcl4 summary of redox reaction. Get your questions answered by the expert for free. Lead (ii) chloride reacts with cl2 to give pbcl4. (a) lead (ii) chloride reacts with cl 2 to give pbcl 4. Pbcl2 + cl2 = pbcl4 is a redox reaction where pb is oxidized and cl is reduced. (b) lead (iv) chloride is highly unstable. Lead Chloride Reacts With Cl2 To Give Pbcl4.