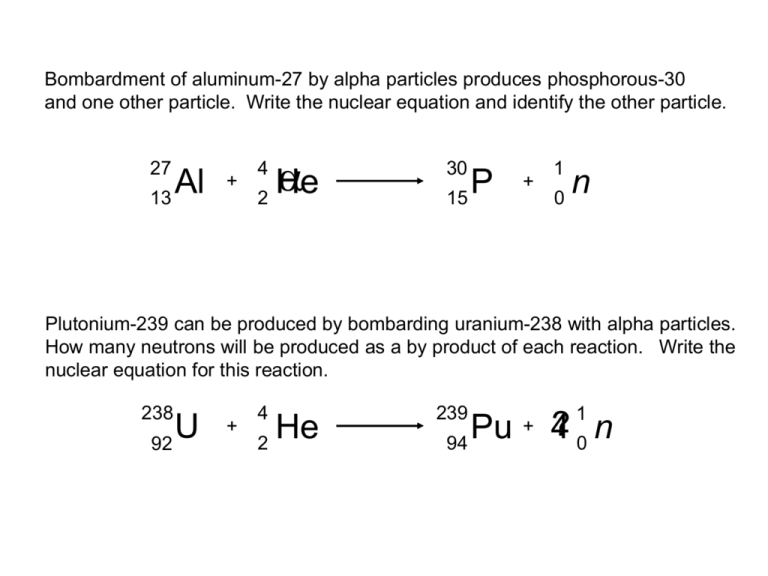
Uranium 238 Undergoes Alpha Decay . The nuclear equation for this. The alpha particle removes two. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The nuclear disintegration process that emits alpha particles is called alpha decay. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium.
from www.tessshebaylo.com
The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. The alpha particle removes two. The nuclear equation for this. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The nuclear disintegration process that emits alpha particles is called alpha decay.
Uranium 238 Radioactive Decay Equation Tessshebaylo
Uranium 238 Undergoes Alpha Decay It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The nuclear disintegration process that emits alpha particles is called alpha decay. The nuclear equation for this. The alpha particle removes two. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less.
From www.chegg.com
Solved The isotope of uranium, 238/92U, undergoes alpha Uranium 238 Undergoes Alpha Decay It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The nuclear equation for this. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. The nuclear disintegration process that emits alpha particles is called alpha decay. The alpha particle removes two. Uranium 238 Undergoes Alpha Decay.
From www.numerade.com
Plutonium242 decays to uranium 238 by emission of an αparticle with Uranium 238 Undergoes Alpha Decay The nuclear disintegration process that emits alpha particles is called alpha decay. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. The nuclear equation for this. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The alpha particle removes two. Uranium 238 Undergoes Alpha Decay.
From www.numerade.com
SOLVEDCalculate the energy released, in joules per mole, when uranium Uranium 238 Undergoes Alpha Decay The nuclear equation for this. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. The nuclear disintegration process that emits alpha particles is called alpha decay. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The alpha particle removes two. Uranium 238 Undergoes Alpha Decay.
From solvedlib.com
15. Complete the nuclear reaction Uranium238 underg… SolvedLib Uranium 238 Undergoes Alpha Decay The nuclear equation for this. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. The nuclear disintegration process that emits alpha particles is called alpha decay. The alpha particle removes two. Uranium 238 Undergoes Alpha Decay.
From www.coursehero.com
[Solved] If Uranium238 undergoes alpha decay what element is produced Uranium 238 Undergoes Alpha Decay The alpha particle removes two. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. The nuclear disintegration process that emits alpha particles is called alpha decay. The nuclear equation for this. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. Uranium 238 Undergoes Alpha Decay.
From www.chegg.com
3. Uranium238 undergoes radioactive decay until a Uranium 238 Undergoes Alpha Decay The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. The nuclear equation for this. The alpha particle removes two. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The nuclear disintegration process that emits alpha particles is called alpha decay. Uranium 238 Undergoes Alpha Decay.
From www.chegg.com
Solved a (1 point) U 238 + Th234 92 A Uranium238 nucleus Uranium 238 Undergoes Alpha Decay It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The nuclear disintegration process that emits alpha particles is called alpha decay. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. The alpha particle removes two. The nuclear equation for this. Uranium 238 Undergoes Alpha Decay.
From www.slideserve.com
PPT Nuclear Energy PowerPoint Presentation, free download ID3222690 Uranium 238 Undergoes Alpha Decay The nuclear disintegration process that emits alpha particles is called alpha decay. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The alpha particle removes two. The nuclear equation for this. Uranium 238 Undergoes Alpha Decay.
From www.slideshare.net
Nuclear Decay Uranium 238 Undergoes Alpha Decay The alpha particle removes two. The nuclear disintegration process that emits alpha particles is called alpha decay. The nuclear equation for this. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. Uranium 238 Undergoes Alpha Decay.
From www.tessshebaylo.com
Uranium 238 Alpha Decay Equation Tessshebaylo Uranium 238 Undergoes Alpha Decay The alpha particle removes two. The nuclear equation for this. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The nuclear disintegration process that emits alpha particles is called alpha decay. Uranium 238 Undergoes Alpha Decay.
From www.chegg.com
3. Uranium238 undergoes radioactive decay until a Uranium 238 Undergoes Alpha Decay It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The alpha particle removes two. The nuclear equation for this. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. The nuclear disintegration process that emits alpha particles is called alpha decay. Uranium 238 Undergoes Alpha Decay.
From www.tessshebaylo.com
Uranium 238 Radioactive Decay Equation Tessshebaylo Uranium 238 Undergoes Alpha Decay The alpha particle removes two. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. The nuclear disintegration process that emits alpha particles is called alpha decay. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The nuclear equation for this. Uranium 238 Undergoes Alpha Decay.
From sciencedrill.com
A Guide to Alpha Decay Including Equations and Uses Science Drill Uranium 238 Undergoes Alpha Decay The nuclear disintegration process that emits alpha particles is called alpha decay. The nuclear equation for this. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. The alpha particle removes two. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. Uranium 238 Undergoes Alpha Decay.
From www.shalom-education.com
Nuclear Decay Equations GCSE Physics Revision Uranium 238 Undergoes Alpha Decay The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. The alpha particle removes two. The nuclear equation for this. The nuclear disintegration process that emits alpha particles is called alpha decay. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. Uranium 238 Undergoes Alpha Decay.
From www.bartleby.com
Answered When uranium238 undergoes alpha decay,… bartleby Uranium 238 Undergoes Alpha Decay The nuclear equation for this. The nuclear disintegration process that emits alpha particles is called alpha decay. The alpha particle removes two. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. Uranium 238 Undergoes Alpha Decay.
From www.rankred.com
What is Alpha Decay? Definition Equation Example RankRed Uranium 238 Undergoes Alpha Decay It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The alpha particle removes two. The nuclear disintegration process that emits alpha particles is called alpha decay. The nuclear equation for this. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. Uranium 238 Undergoes Alpha Decay.
From brainly.com
Uranium238 undergoes alpha decay. In addition to the alpha particle Uranium 238 Undergoes Alpha Decay The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. The nuclear disintegration process that emits alpha particles is called alpha decay. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The nuclear equation for this. The alpha particle removes two. Uranium 238 Undergoes Alpha Decay.
From www.nachi.org
Uranium238 Decay Chain Inspection Gallery InterNACHI® Uranium 238 Undergoes Alpha Decay The nuclear disintegration process that emits alpha particles is called alpha decay. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. The alpha particle removes two. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The nuclear equation for this. Uranium 238 Undergoes Alpha Decay.
From www.researchgate.net
Uranium238 radioactive chain diagram. The radioactive chain begins Uranium 238 Undergoes Alpha Decay The nuclear equation for this. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The alpha particle removes two. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. The nuclear disintegration process that emits alpha particles is called alpha decay. Uranium 238 Undergoes Alpha Decay.
From www.numerade.com
SOLVED A uranium nucleus (mass 238 u), initially at rest, undergoes Uranium 238 Undergoes Alpha Decay It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The nuclear equation for this. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. The nuclear disintegration process that emits alpha particles is called alpha decay. The alpha particle removes two. Uranium 238 Undergoes Alpha Decay.
From www.youtube.com
Physical Science 7.4f The Decay of Uranium YouTube Uranium 238 Undergoes Alpha Decay The alpha particle removes two. The nuclear equation for this. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The nuclear disintegration process that emits alpha particles is called alpha decay. Uranium 238 Undergoes Alpha Decay.
From www.chegg.com
Solved A uranium238 nucleus at rest undergoes radioactive Uranium 238 Undergoes Alpha Decay The nuclear disintegration process that emits alpha particles is called alpha decay. The nuclear equation for this. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The alpha particle removes two. Uranium 238 Undergoes Alpha Decay.
From general.chemistrysteps.com
Balancing Nuclear Reactions Chemistry Steps Uranium 238 Undergoes Alpha Decay The nuclear equation for this. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. The nuclear disintegration process that emits alpha particles is called alpha decay. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The alpha particle removes two. Uranium 238 Undergoes Alpha Decay.
From www.tessshebaylo.com
Uranium 238 Radioactive Decay Equation Tessshebaylo Uranium 238 Undergoes Alpha Decay It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The nuclear equation for this. The alpha particle removes two. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. The nuclear disintegration process that emits alpha particles is called alpha decay. Uranium 238 Undergoes Alpha Decay.
From www.slideserve.com
PPT Chapter 10 Nuclear Chemistry PowerPoint Presentation, free Uranium 238 Undergoes Alpha Decay The nuclear disintegration process that emits alpha particles is called alpha decay. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The alpha particle removes two. The nuclear equation for this. Uranium 238 Undergoes Alpha Decay.
From www.expii.com
Alpha Decay — Definition & Overview Expii Uranium 238 Undergoes Alpha Decay The nuclear disintegration process that emits alpha particles is called alpha decay. The alpha particle removes two. The nuclear equation for this. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. Uranium 238 Undergoes Alpha Decay.
From www.chegg.com
Solved Uranium238 undergoes radioactive decay until a Uranium 238 Undergoes Alpha Decay The nuclear equation for this. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. The nuclear disintegration process that emits alpha particles is called alpha decay. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The alpha particle removes two. Uranium 238 Undergoes Alpha Decay.
From www.researchgate.net
Decay series of (A) uranium238 ( 238 U), (B) uranium235 ( 235 U), and Uranium 238 Undergoes Alpha Decay The alpha particle removes two. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The nuclear disintegration process that emits alpha particles is called alpha decay. The nuclear equation for this. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. Uranium 238 Undergoes Alpha Decay.
From socratic.org
What is the product produced when ""_92^238"U" undergoes alpha decay Uranium 238 Undergoes Alpha Decay The nuclear disintegration process that emits alpha particles is called alpha decay. The alpha particle removes two. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The nuclear equation for this. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. Uranium 238 Undergoes Alpha Decay.
From www.radiation-dosimetry.org
What is Alpha Decay Alpha Radioactivity Definition Uranium 238 Undergoes Alpha Decay The nuclear equation for this. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. The nuclear disintegration process that emits alpha particles is called alpha decay. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The alpha particle removes two. Uranium 238 Undergoes Alpha Decay.
From www.epa.gov
Radioactive Decay US EPA Uranium 238 Undergoes Alpha Decay The alpha particle removes two. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. The nuclear equation for this. The nuclear disintegration process that emits alpha particles is called alpha decay. Uranium 238 Undergoes Alpha Decay.
From www.slideserve.com
PPT Introduction PowerPoint Presentation, free download ID5758308 Uranium 238 Undergoes Alpha Decay It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The nuclear disintegration process that emits alpha particles is called alpha decay. The alpha particle removes two. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. The nuclear equation for this. Uranium 238 Undergoes Alpha Decay.
From www.chegg.com
Solved Uranium238 undergoes radioactive decay until a Uranium 238 Undergoes Alpha Decay It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The nuclear disintegration process that emits alpha particles is called alpha decay. The alpha particle removes two. The nuclear equation for this. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. Uranium 238 Undergoes Alpha Decay.
From www.researchgate.net
Uranium238 decay chain from Wikipedia under CClicence. Download Uranium 238 Undergoes Alpha Decay It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The nuclear equation for this. The alpha particle removes two. The nuclear disintegration process that emits alpha particles is called alpha decay. The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. Uranium 238 Undergoes Alpha Decay.
From www.youtube.com
How many alpha and beta particles are emitted when uranium `._(92)^(238 Uranium 238 Undergoes Alpha Decay The alpha particle removes two protons (green) and two neutrons (gray) from the uranium. The alpha particle removes two. The nuclear disintegration process that emits alpha particles is called alpha decay. It transforms (or decays) into an atom with an atomic number 2 less and a mass number 4 less. The nuclear equation for this. Uranium 238 Undergoes Alpha Decay.