Why Is Ca2 Smaller Than Ca . Nax+ n a x + and cax+ c a x + have thus similar radii as well but, when. cations are always smaller than the neutral atom and anions are always larger. Rather, the ca ion is in a net +2. Packing of ions in ionic lattices, and therefore, the lattice energy. a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. — sizes of ions influence: — according to the diagonal relation, na n a and ca c a have similar radii. — all five ions have an equal number of electrons and therefore share the same electron configuration. — however, that's not the case— both the ion and the neutral atom have 20 pos. — for a) the neutral ca atom has a larger radius than the ca2+ ion. This is because the loss of electrons reduces the shielding effect between. Because most elements form either a cation or an anion but not both, there.
from forum.facmedicine.com
Packing of ions in ionic lattices, and therefore, the lattice energy. This is because the loss of electrons reduces the shielding effect between. — sizes of ions influence: — however, that's not the case— both the ion and the neutral atom have 20 pos. — according to the diagonal relation, na n a and ca c a have similar radii. a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. Because most elements form either a cation or an anion but not both, there. Nax+ n a x + and cax+ c a x + have thus similar radii as well but, when. — all five ions have an equal number of electrons and therefore share the same electron configuration. — for a) the neutral ca atom has a larger radius than the ca2+ ion.
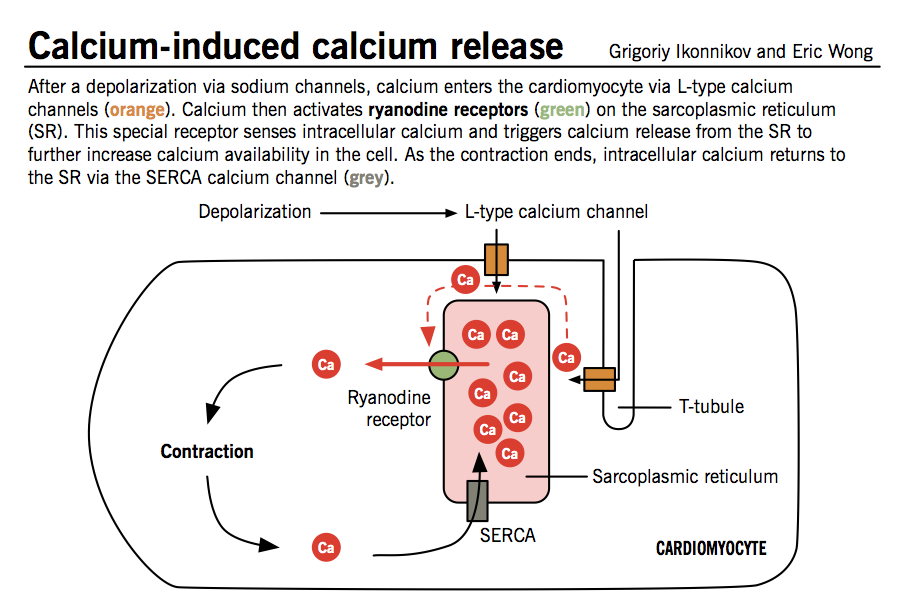
Physiology Of Cardiac Conduction And Contractility Faculty of Medicine
Why Is Ca2 Smaller Than Ca — however, that's not the case— both the ion and the neutral atom have 20 pos. a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. Rather, the ca ion is in a net +2. This is because the loss of electrons reduces the shielding effect between. Packing of ions in ionic lattices, and therefore, the lattice energy. cations are always smaller than the neutral atom and anions are always larger. Because most elements form either a cation or an anion but not both, there. — for a) the neutral ca atom has a larger radius than the ca2+ ion. — according to the diagonal relation, na n a and ca c a have similar radii. Nax+ n a x + and cax+ c a x + have thus similar radii as well but, when. — however, that's not the case— both the ion and the neutral atom have 20 pos. — sizes of ions influence: — all five ions have an equal number of electrons and therefore share the same electron configuration.
From www.researchgate.net
CaMKKmediated cellular signaling. Increasing intracellular Ca 2 Why Is Ca2 Smaller Than Ca Nax+ n a x + and cax+ c a x + have thus similar radii as well but, when. — all five ions have an equal number of electrons and therefore share the same electron configuration. Packing of ions in ionic lattices, and therefore, the lattice energy. Because most elements form either a cation or an anion but not. Why Is Ca2 Smaller Than Ca.
From www.pinterest.com
Ca 2+ Electron Configuration (Calcium Ion) Electron configuration Why Is Ca2 Smaller Than Ca — for a) the neutral ca atom has a larger radius than the ca2+ ion. — sizes of ions influence: — all five ions have an equal number of electrons and therefore share the same electron configuration. This is because the loss of electrons reduces the shielding effect between. — however, that's not the case— both. Why Is Ca2 Smaller Than Ca.
From forum.facmedicine.com
Physiology Of Cardiac Conduction And Contractility Faculty of Medicine Why Is Ca2 Smaller Than Ca Packing of ions in ionic lattices, and therefore, the lattice energy. — for a) the neutral ca atom has a larger radius than the ca2+ ion. — sizes of ions influence: This is because the loss of electrons reduces the shielding effect between. Rather, the ca ion is in a net +2. — all five ions have. Why Is Ca2 Smaller Than Ca.
From www.mdpi.com
IJMS Free FullText Systematic Review of Calcium Channels and Why Is Ca2 Smaller Than Ca — sizes of ions influence: cations are always smaller than the neutral atom and anions are always larger. — all five ions have an equal number of electrons and therefore share the same electron configuration. — according to the diagonal relation, na n a and ca c a have similar radii. — for a) the. Why Is Ca2 Smaller Than Ca.
From www.researchgate.net
Calcium homeostasis in normal cells. Ionized calcium (Ca 2 + ) in the Why Is Ca2 Smaller Than Ca — however, that's not the case— both the ion and the neutral atom have 20 pos. Because most elements form either a cation or an anion but not both, there. — for a) the neutral ca atom has a larger radius than the ca2+ ion. This is because the loss of electrons reduces the shielding effect between. . Why Is Ca2 Smaller Than Ca.
From slideplayer.com
Chemical Bonding part ppt download Why Is Ca2 Smaller Than Ca a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. Rather, the ca ion is in a net +2. — however, that's not the case— both the ion and the neutral atom have 20 pos. cations are always smaller than the neutral atom and anions. Why Is Ca2 Smaller Than Ca.
From cvphysiology.com
CV Physiology SodiumCalcium Exchange in Cardiac Cells Why Is Ca2 Smaller Than Ca — however, that's not the case— both the ion and the neutral atom have 20 pos. Nax+ n a x + and cax+ c a x + have thus similar radii as well but, when. a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. . Why Is Ca2 Smaller Than Ca.
From slideplayer.com
Elemental Properties and Patterns ppt download Why Is Ca2 Smaller Than Ca a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. — sizes of ions influence: cations are always smaller than the neutral atom and anions are always larger. Nax+ n a x + and cax+ c a x + have thus similar radii as well. Why Is Ca2 Smaller Than Ca.
From www.mdpi.com
Cells Free FullText Calcium Channels in the Heart Disease States Why Is Ca2 Smaller Than Ca cations are always smaller than the neutral atom and anions are always larger. Because most elements form either a cation or an anion but not both, there. — according to the diagonal relation, na n a and ca c a have similar radii. — all five ions have an equal number of electrons and therefore share the. Why Is Ca2 Smaller Than Ca.
From www.youtube.com
Ca 2+ Electron Configuration (Calcium Ion) YouTube Why Is Ca2 Smaller Than Ca — for a) the neutral ca atom has a larger radius than the ca2+ ion. — all five ions have an equal number of electrons and therefore share the same electron configuration. Rather, the ca ion is in a net +2. This is because the loss of electrons reduces the shielding effect between. Because most elements form either. Why Is Ca2 Smaller Than Ca.
From labpedia.net
blood coagulation factors and interpretations Why Is Ca2 Smaller Than Ca cations are always smaller than the neutral atom and anions are always larger. a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. — all five ions have an equal number of electrons and therefore share the same electron configuration. This is because the loss. Why Is Ca2 Smaller Than Ca.
From www.frontiersin.org
Frontiers The Mitochondrial Ca2+ Uptake and the of Why Is Ca2 Smaller Than Ca Nax+ n a x + and cax+ c a x + have thus similar radii as well but, when. Rather, the ca ion is in a net +2. — for a) the neutral ca atom has a larger radius than the ca2+ ion. — according to the diagonal relation, na n a and ca c a have similar. Why Is Ca2 Smaller Than Ca.
From studybreathings.z21.web.core.windows.net
How To Draw Electron Configuration Why Is Ca2 Smaller Than Ca Because most elements form either a cation or an anion but not both, there. a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. — according to the diagonal relation, na n a and ca c a have similar radii. — sizes of ions influence:. Why Is Ca2 Smaller Than Ca.
From www.ahajournals.org
Inherited Dysfunction of Sarcoplasmic Reticulum Ca2+ Handling and Why Is Ca2 Smaller Than Ca Packing of ions in ionic lattices, and therefore, the lattice energy. — sizes of ions influence: This is because the loss of electrons reduces the shielding effect between. — according to the diagonal relation, na n a and ca c a have similar radii. — all five ions have an equal number of electrons and therefore share. Why Is Ca2 Smaller Than Ca.
From valenceelectrons.com
Electron Configuration for Calcium (Ca, Ca2+ ion) Why Is Ca2 Smaller Than Ca — sizes of ions influence: — however, that's not the case— both the ion and the neutral atom have 20 pos. Packing of ions in ionic lattices, and therefore, the lattice energy. Because most elements form either a cation or an anion but not both, there. This is because the loss of electrons reduces the shielding effect between.. Why Is Ca2 Smaller Than Ca.
From www.youtube.com
Which is larger? Ca or Ca 2+ (Calcium atom or Calcium ion) YouTube Why Is Ca2 Smaller Than Ca Nax+ n a x + and cax+ c a x + have thus similar radii as well but, when. Because most elements form either a cation or an anion but not both, there. Rather, the ca ion is in a net +2. Packing of ions in ionic lattices, and therefore, the lattice energy. — sizes of ions influence: . Why Is Ca2 Smaller Than Ca.
From www.researchgate.net
The charge of Ca 2+ ions released by CCF plotted as a function of the Why Is Ca2 Smaller Than Ca a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. Packing of ions in ionic lattices, and therefore, the lattice energy. Nax+ n a x + and cax+ c a x + have thus similar radii as well but, when. — for a) the neutral ca. Why Is Ca2 Smaller Than Ca.
From slideplayer.com
Periodic Properties of the Elements ppt download Why Is Ca2 Smaller Than Ca Nax+ n a x + and cax+ c a x + have thus similar radii as well but, when. a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. — all five ions have an equal number of electrons and therefore share the same electron configuration.. Why Is Ca2 Smaller Than Ca.
From journals.sagepub.com
Sarcoidosis and calcium homeostasis disturbances—Do we know where we Why Is Ca2 Smaller Than Ca — sizes of ions influence: Packing of ions in ionic lattices, and therefore, the lattice energy. This is because the loss of electrons reduces the shielding effect between. cations are always smaller than the neutral atom and anions are always larger. a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is. Why Is Ca2 Smaller Than Ca.
From www.researchgate.net
Ca 2+ versus Li + competition in the trinuclear Ca 2+ site of Syt7 C2a Why Is Ca2 Smaller Than Ca — however, that's not the case— both the ion and the neutral atom have 20 pos. — according to the diagonal relation, na n a and ca c a have similar radii. a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. cations are. Why Is Ca2 Smaller Than Ca.
From dxoxivnee.blob.core.windows.net
Signal Transduction Pathway Simple at Cathleen Johnson blog Why Is Ca2 Smaller Than Ca cations are always smaller than the neutral atom and anions are always larger. — however, that's not the case— both the ion and the neutral atom have 20 pos. Rather, the ca ion is in a net +2. — for a) the neutral ca atom has a larger radius than the ca2+ ion. Because most elements form. Why Is Ca2 Smaller Than Ca.
From www.researchgate.net
Wnt/Ca2+ signalling pathway shows calcium at different compartments Why Is Ca2 Smaller Than Ca Nax+ n a x + and cax+ c a x + have thus similar radii as well but, when. Because most elements form either a cation or an anion but not both, there. — sizes of ions influence: Rather, the ca ion is in a net +2. Packing of ions in ionic lattices, and therefore, the lattice energy. . Why Is Ca2 Smaller Than Ca.
From circuitlibraryspaes.z13.web.core.windows.net
Electron Dot Diagram Calcium Why Is Ca2 Smaller Than Ca Nax+ n a x + and cax+ c a x + have thus similar radii as well but, when. — according to the diagonal relation, na n a and ca c a have similar radii. — for a) the neutral ca atom has a larger radius than the ca2+ ion. Because most elements form either a cation or. Why Is Ca2 Smaller Than Ca.
From chemistry.stackexchange.com
atomic radius Why does Mg2+ have the ionic radii bigger than Ca2 Why Is Ca2 Smaller Than Ca cations are always smaller than the neutral atom and anions are always larger. This is because the loss of electrons reduces the shielding effect between. a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. Because most elements form either a cation or an anion but. Why Is Ca2 Smaller Than Ca.
From www.chegg.com
Solved Predict the equilibrium concentration of Ca2+ in the Why Is Ca2 Smaller Than Ca — all five ions have an equal number of electrons and therefore share the same electron configuration. Because most elements form either a cation or an anion but not both, there. — however, that's not the case— both the ion and the neutral atom have 20 pos. — sizes of ions influence: — for a) the. Why Is Ca2 Smaller Than Ca.
From www.numerade.com
SOLVED The ionic radius of the chloride ion (Cl) is 167 pm and the Why Is Ca2 Smaller Than Ca — all five ions have an equal number of electrons and therefore share the same electron configuration. Rather, the ca ion is in a net +2. — sizes of ions influence: — for a) the neutral ca atom has a larger radius than the ca2+ ion. — according to the diagonal relation, na n a and. Why Is Ca2 Smaller Than Ca.
From www.mdpi.com
Membranes Free FullText Molecular Choreography and Structure of Why Is Ca2 Smaller Than Ca cations are always smaller than the neutral atom and anions are always larger. a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. This is because the loss of electrons reduces the shielding effect between. — however, that's not the case— both the ion and. Why Is Ca2 Smaller Than Ca.
From pressbooks.rampages.us
Calcium Critical for Bones and Throughout the Body Nutrition Why Is Ca2 Smaller Than Ca — according to the diagonal relation, na n a and ca c a have similar radii. — however, that's not the case— both the ion and the neutral atom have 20 pos. — all five ions have an equal number of electrons and therefore share the same electron configuration. cations are always smaller than the neutral. Why Is Ca2 Smaller Than Ca.
From www.chegg.com
Solved Explain why a) Calcium forms only Ca2+ compounds, Why Is Ca2 Smaller Than Ca — sizes of ions influence: Packing of ions in ionic lattices, and therefore, the lattice energy. a comparison of ionic radii with atomic radii (figure 2.8.7) cation, having lost an electron, is always smaller than its parent neutral. — for a) the neutral ca atom has a larger radius than the ca2+ ion. cations are always. Why Is Ca2 Smaller Than Ca.
From studyx.ai
Which particle represents the size of the StudyX Why Is Ca2 Smaller Than Ca Nax+ n a x + and cax+ c a x + have thus similar radii as well but, when. — sizes of ions influence: cations are always smaller than the neutral atom and anions are always larger. Rather, the ca ion is in a net +2. a comparison of ionic radii with atomic radii (figure 2.8.7) cation,. Why Is Ca2 Smaller Than Ca.
From www.researchgate.net
Energy level diagram showing the levels of Ca, Ca + and Ca 2+ of Why Is Ca2 Smaller Than Ca — however, that's not the case— both the ion and the neutral atom have 20 pos. cations are always smaller than the neutral atom and anions are always larger. Because most elements form either a cation or an anion but not both, there. Rather, the ca ion is in a net +2. — sizes of ions influence:. Why Is Ca2 Smaller Than Ca.
From mavink.com
Calcium Orbital Diagram Why Is Ca2 Smaller Than Ca Rather, the ca ion is in a net +2. cations are always smaller than the neutral atom and anions are always larger. Packing of ions in ionic lattices, and therefore, the lattice energy. — all five ions have an equal number of electrons and therefore share the same electron configuration. — according to the diagonal relation, na. Why Is Ca2 Smaller Than Ca.
From www.pnas.org
Increased intracellular Ca2+ concentrations prevent membrane Why Is Ca2 Smaller Than Ca — for a) the neutral ca atom has a larger radius than the ca2+ ion. cations are always smaller than the neutral atom and anions are always larger. — according to the diagonal relation, na n a and ca c a have similar radii. Rather, the ca ion is in a net +2. — sizes of. Why Is Ca2 Smaller Than Ca.
From www.youtube.com
How to find Protons & Electrons for the Calcium ion (Ca 2+) YouTube Why Is Ca2 Smaller Than Ca Packing of ions in ionic lattices, and therefore, the lattice energy. — according to the diagonal relation, na n a and ca c a have similar radii. — however, that's not the case— both the ion and the neutral atom have 20 pos. This is because the loss of electrons reduces the shielding effect between. a comparison. Why Is Ca2 Smaller Than Ca.
From exomqzeef.blob.core.windows.net
Is Um Smaller Than Nm at Gemma Diaz blog Why Is Ca2 Smaller Than Ca cations are always smaller than the neutral atom and anions are always larger. — according to the diagonal relation, na n a and ca c a have similar radii. Rather, the ca ion is in a net +2. This is because the loss of electrons reduces the shielding effect between. — however, that's not the case— both. Why Is Ca2 Smaller Than Ca.