What Is Mdr Eu . the european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. Medical devices are products or equipment intended for a medical purpose. It updates the rules on. In the european union (eu). the mdr certification is required for medical device manufacturers to legally market and sell their products in the eu. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for. What is the aim of the regulation? regulation (eu) 2017/745 on medical devices. the new medical devices regulation (2017/745/ eu) (mdr) and the new in vitro diagnostic medical devices regulation.
from clin-r.com
regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. the european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. It updates the rules on. the mdr certification is required for medical device manufacturers to legally market and sell their products in the eu. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for. regulation (eu) 2017/745 on medical devices. In the european union (eu). the new medical devices regulation (2017/745/ eu) (mdr) and the new in vitro diagnostic medical devices regulation. Medical devices are products or equipment intended for a medical purpose. What is the aim of the regulation?
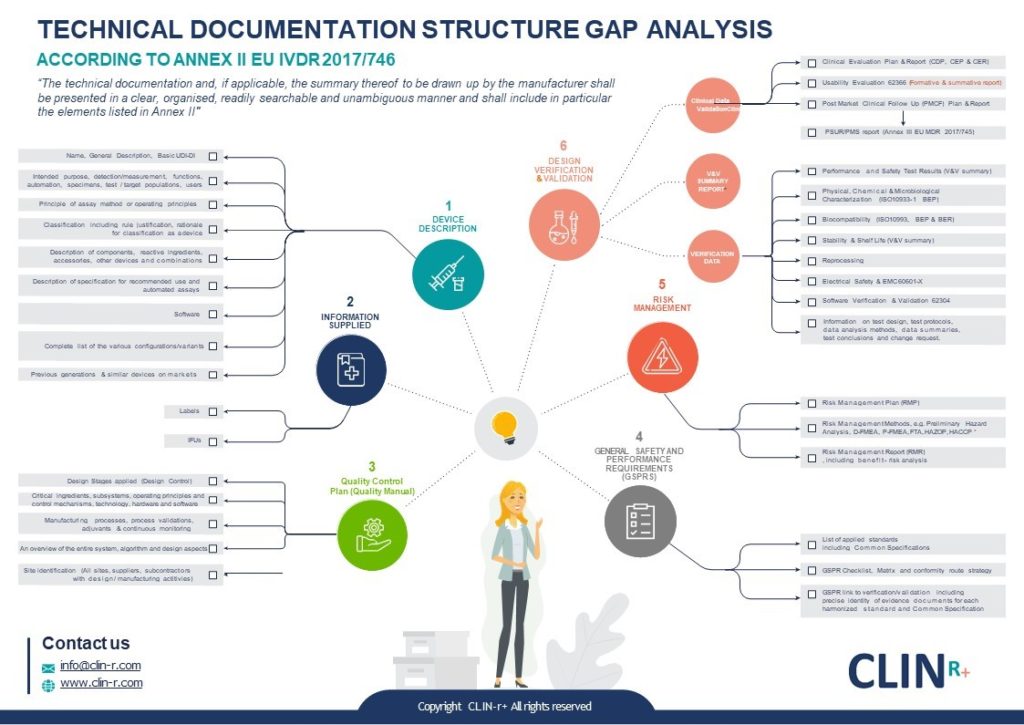
EU MDR how to structure your Medical Device Technical Document Clin R
What Is Mdr Eu the mdr certification is required for medical device manufacturers to legally market and sell their products in the eu. Medical devices are products or equipment intended for a medical purpose. the new medical devices regulation (2017/745/ eu) (mdr) and the new in vitro diagnostic medical devices regulation. It updates the rules on. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for. the mdr certification is required for medical device manufacturers to legally market and sell their products in the eu. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. the european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. What is the aim of the regulation? regulation (eu) 2017/745 on medical devices. In the european union (eu).
From connectorsupplier.com
EU MDR Update to Medical Device Regulations in Europe What Is Mdr Eu regulation (eu) 2017/745 on medical devices. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. In the european union (eu). What is the aim of the regulation? the european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official. What Is Mdr Eu.
From ceqcidjn.blob.core.windows.net
Eu Mdr Medical Device Labeling Requirements at Mary Plank blog What Is Mdr Eu What is the aim of the regulation? In the european union (eu). regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. the european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. It updates the. What Is Mdr Eu.
From medrio.com
European MDR (EU MDR) Guide to Prepare What Is Mdr Eu In the european union (eu). the medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for. the new medical devices regulation (2017/745/ eu) (mdr) and the new in vitro diagnostic medical devices regulation. the mdr certification is required for medical device manufacturers to legally market and sell their products in the. What Is Mdr Eu.
From www.reliantlifesciences.com
EU MDR Timeline Reliant Life What Is Mdr Eu the medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for. the european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. regulation (eu) 2017/745 on medical devices. the new medical devices regulation (2017/745/ eu) (mdr) and the. What Is Mdr Eu.
From platohealth.ai
Ultimate Guide To Device Class Requirements Under EU MDR PlatoHealth What Is Mdr Eu the new medical devices regulation (2017/745/ eu) (mdr) and the new in vitro diagnostic medical devices regulation. It updates the rules on. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for. Medical devices are products or equipment intended for a medical purpose. regulation (eu) 2017/745 of the european parliament. What Is Mdr Eu.
From omcmedical.com
Quality Management System Requirement of EU MDR OMC Medical What Is Mdr Eu regulation (eu) 2017/745 on medical devices. It updates the rules on. What is the aim of the regulation? Medical devices are products or equipment intended for a medical purpose. In the european union (eu). the european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. . What Is Mdr Eu.
From www.greenlight.guru
EU MDR 9 Top Questions Answered (European MDR) What Is Mdr Eu the medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. In the european union (eu). regulation (eu) 2017/745 on medical devices. the new medical devices regulation (2017/745/ eu) (mdr). What Is Mdr Eu.
From advisera.com
EU MDR vs UK MDR What Is Mdr Eu regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. It updates the rules on. Medical devices are products or equipment intended for a medical purpose. regulation (eu) 2017/745 on medical devices. the mdr certification is required for medical device manufacturers to legally market and sell their. What Is Mdr Eu.
From www.scilife.io
EU MDR Key Changes and Important Steps Scilife What Is Mdr Eu regulation (eu) 2017/745 on medical devices. It updates the rules on. What is the aim of the regulation? In the european union (eu). Medical devices are products or equipment intended for a medical purpose. the mdr certification is required for medical device manufacturers to legally market and sell their products in the eu. the new medical devices. What Is Mdr Eu.
From advisera.com
What is EU MDR? Advisera What Is Mdr Eu the mdr certification is required for medical device manufacturers to legally market and sell their products in the eu. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. It. What Is Mdr Eu.
From rs-ness.com
Preparing your Quality Management System for the EU MDR What Is Mdr Eu In the european union (eu). regulation (eu) 2017/745 on medical devices. the new medical devices regulation (2017/745/ eu) (mdr) and the new in vitro diagnostic medical devices regulation. the european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. It updates the rules on. . What Is Mdr Eu.
From www.congress-intercultural.eu
What Is The EU MDR? Indepth Explanation Of The Regulation, 52 OFF What Is Mdr Eu the medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for. the new medical devices regulation (2017/745/ eu) (mdr) and the new in vitro diagnostic medical devices regulation. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. What is the. What Is Mdr Eu.
From www.mastercontrol.com
6 Points for Successfully Navigating the EU MDR MasterControl What Is Mdr Eu regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. In the european union (eu). the new medical devices regulation (2017/745/ eu) (mdr) and the new in vitro diagnostic medical devices regulation. It updates the rules on. the mdr certification is required for medical device manufacturers to. What Is Mdr Eu.
From cemarking.net
EU MDR 2017/745 What Changed? What Is Mdr Eu In the european union (eu). Medical devices are products or equipment intended for a medical purpose. What is the aim of the regulation? regulation (eu) 2017/745 on medical devices. It updates the rules on. the mdr certification is required for medical device manufacturers to legally market and sell their products in the eu. the medical device regulation. What Is Mdr Eu.
From www.meddeviceonline.com
Infographic EU MDR Is the Industry Planning for It Are You What Is Mdr Eu the medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for. the new medical devices regulation (2017/745/ eu) (mdr) and the new in vitro diagnostic medical devices regulation. regulation (eu) 2017/745 on medical devices. Medical devices are products or equipment intended for a medical purpose. What is the aim of the. What Is Mdr Eu.
From www.acquiscompliance.com
EU MDR Compliance Key Requirements for Medical Devices What Is Mdr Eu It updates the rules on. What is the aim of the regulation? the medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for. the european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. regulation (eu) 2017/745 on medical. What Is Mdr Eu.
From www.mylanguageconnection.com
The EU Medical Device Regulation [EU MDR] My Language Connection What Is Mdr Eu In the european union (eu). the new medical devices regulation (2017/745/ eu) (mdr) and the new in vitro diagnostic medical devices regulation. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices,. What Is Mdr Eu.
From www.hydrix.com
Conquering the EU MDR/IVDR with a welldefined regulatory strategy What Is Mdr Eu regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. It updates the rules on. the new medical devices regulation (2017/745/ eu) (mdr) and the new in vitro diagnostic medical devices regulation. Medical devices are products or equipment intended for a medical purpose. regulation (eu) 2017/745 on. What Is Mdr Eu.
From www.regdesk.co
EU MDR overview An Update to European Medical Device Regulations RegDesk What Is Mdr Eu the mdr certification is required for medical device manufacturers to legally market and sell their products in the eu. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. In. What Is Mdr Eu.
From www.assurx.com
EU MDR Countdown What's Next for Medical Device Compliance AssurX What Is Mdr Eu regulation (eu) 2017/745 on medical devices. In the european union (eu). the medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for. Medical devices are products or equipment intended for a medical purpose. What is the aim of the regulation? regulation (eu) 2017/745 of the european parliament and of the council. What Is Mdr Eu.
From www.regulatoryglobe.com
EU MDR implementation guide for medical devices MDCG What Is Mdr Eu the european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for. the mdr certification is required for medical device manufacturers to legally market and sell their products in. What Is Mdr Eu.
From kobridgeconsulting.com
What is the new EU MDR what does it mean for manufacturers Kobridge What Is Mdr Eu regulation (eu) 2017/745 on medical devices. the mdr certification is required for medical device manufacturers to legally market and sell their products in the eu. It updates the rules on. What is the aim of the regulation? the european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal. What Is Mdr Eu.
From www.youtube.com
The EU MDR Transition Period Extension What we know so far and what to What Is Mdr Eu the european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. the mdr certification is required for medical device manufacturers to legally market and sell their products in the eu. It updates the rules on. the new medical devices regulation (2017/745/ eu) (mdr) and the. What Is Mdr Eu.
From www.extrahorizon.com
What does the EU MDR mean for your medical device product? What Is Mdr Eu the mdr certification is required for medical device manufacturers to legally market and sell their products in the eu. the new medical devices regulation (2017/745/ eu) (mdr) and the new in vitro diagnostic medical devices regulation. It updates the rules on. regulation (eu) 2017/745 on medical devices. Medical devices are products or equipment intended for a medical. What Is Mdr Eu.
From galtmedical.com
What the EU MDR Extension Means for Medical Device Buyers Galt Medical What Is Mdr Eu Medical devices are products or equipment intended for a medical purpose. the mdr certification is required for medical device manufacturers to legally market and sell their products in the eu. the european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. It updates the rules on.. What Is Mdr Eu.
From www.ecomedics.com
EUMDR Approval ECO MEDICS What Is Mdr Eu the medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for. the european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical. What Is Mdr Eu.
From operonstrategist.com
EU MDR FAQs All you need to know (European MDR) Operon Strategist What Is Mdr Eu In the european union (eu). the new medical devices regulation (2017/745/ eu) (mdr) and the new in vitro diagnostic medical devices regulation. It updates the rules on. regulation (eu) 2017/745 on medical devices. the medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for. the european commission published the first. What Is Mdr Eu.
From naala.nl
What is the difference between the EU MDR and EU IVDR? NAALA What Is Mdr Eu regulation (eu) 2017/745 on medical devices. the mdr certification is required for medical device manufacturers to legally market and sell their products in the eu. Medical devices are products or equipment intended for a medical purpose. In the european union (eu). the european commission published the first implementing regulation related to the eu mdr and eu ivdr. What Is Mdr Eu.
From www.saxocon.com
Get Ready for EU MDR SAXOCON A/S What Is Mdr Eu regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. In the european union (eu). the medical device regulation (mdr), which was adopted in april 2017, changes the european legal framework for. the new medical devices regulation (2017/745/ eu) (mdr) and the new in vitro diagnostic medical. What Is Mdr Eu.
From emmainternational.com
Understanding Annex I of EU MDR What Is Mdr Eu the mdr certification is required for medical device manufacturers to legally market and sell their products in the eu. the european commission published the first implementing regulation related to the eu mdr and eu ivdr in the official journal of the eu. regulation (eu) 2017/745 on medical devices. regulation (eu) 2017/745 of the european parliament and. What Is Mdr Eu.
From www.iascertification.com
EUMDR Certification Medical Device Regulation IAS What Is Mdr Eu the new medical devices regulation (2017/745/ eu) (mdr) and the new in vitro diagnostic medical devices regulation. What is the aim of the regulation? In the european union (eu). the mdr certification is required for medical device manufacturers to legally market and sell their products in the eu. the medical device regulation (mdr), which was adopted in. What Is Mdr Eu.
From www.jamasoftware.com
EU MDR What You Need to Know Jama Software What Is Mdr Eu regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. Medical devices are products or equipment intended for a medical purpose. In the european union (eu). What is the aim of the regulation? the european commission published the first implementing regulation related to the eu mdr and eu. What Is Mdr Eu.
From medrio.com
European MDR (EU MDR) Guide to Prepare What Is Mdr Eu regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices, amending directive. the new medical devices regulation (2017/745/ eu) (mdr) and the new in vitro diagnostic medical devices regulation. regulation (eu) 2017/745 on medical devices. the mdr certification is required for medical device manufacturers to legally market and. What Is Mdr Eu.
From www.congress-intercultural.eu
EU MDR MDD Key Differences [Infographic], 58 OFF What Is Mdr Eu the mdr certification is required for medical device manufacturers to legally market and sell their products in the eu. What is the aim of the regulation? regulation (eu) 2017/745 on medical devices. the new medical devices regulation (2017/745/ eu) (mdr) and the new in vitro diagnostic medical devices regulation. the european commission published the first implementing. What Is Mdr Eu.
From clin-r.com
EU MDR how to structure your Medical Device Technical Document Clin R What Is Mdr Eu the new medical devices regulation (2017/745/ eu) (mdr) and the new in vitro diagnostic medical devices regulation. regulation (eu) 2017/745 on medical devices. It updates the rules on. Medical devices are products or equipment intended for a medical purpose. regulation (eu) 2017/745 of the european parliament and of the council of 5 april 2017 on medical devices,. What Is Mdr Eu.